A Bradford Hill analysis—a set of criteria first proposed by the British epidemiologist Sir Austin Bradford Hill in 1965 to evaluate the strength of evidence for a causal relationship between two variables1 — often plays a critical role in causation opinions of plaintiff experts in product liability matters. While the application of the individual Bradford Hill criteria has faced increasing scrutiny,2 misuse of the overall Bradford Hill framework should not be ignored. Plaintiff experts are increasingly bending traditional Bradford Hill analyses that assess a single association into combined or "shared" analyses that include multiple associations. This article summarizes the use of shared Bradford Hill analyses by the plaintiffs' experts in the In re Acetaminophen litigation, the court's rationale for rejecting that approach, and ramifications of the court's decision for future proceedings.
Shared Bradford Hill Analyses
The Bradford Hill criteria are a set of nine principles used to determine causality between an exposure and an outcome. They include strength of association, temporality, consistency, biological gradient, coherence, biological plausibility, analogy, experimental evidence, and specificity. The framework is not a strict set of rules, and no single criterion, by itself, is enough to establish causality.3 For a more in-depth discussion of the Bradford Hill criteria, see our prior analyses here and here.
The scientific literature is replete with references to the proposition that the Bradford Hill criteria were created to help scientists evaluate a causal association between two variables—typically, an exposure (or another variable) and an outcome.4 Indeed, Sir Hill in his famous 1965 publication applied his nine criteria to assess causality between smoking (the exposure) and lung cancer (the outcome). While other related exposures and outcomes can be considered via certain individual criteria, the overall Bradford Hill framework is intended to evaluate causality for a single association. A diagram illustrating this concept is provided in figure 1 below:
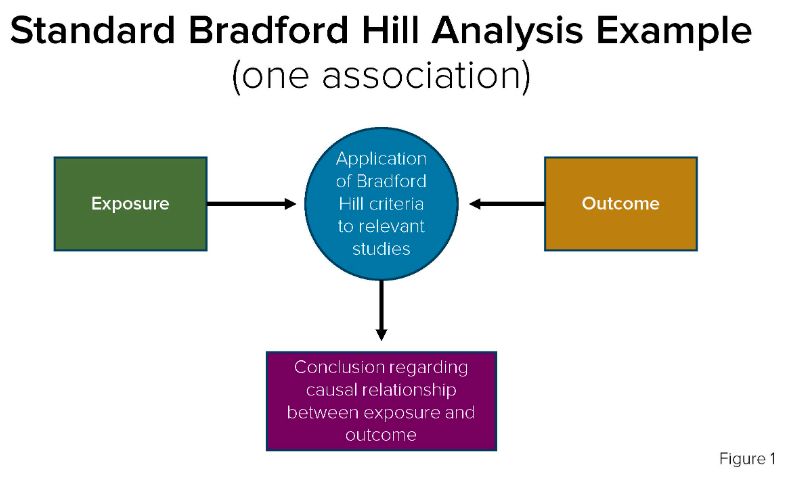 image
image
However, with growing frequency, plaintiff experts in product liability proceedings have been employing shared Bradford Hill analyses that combine several exposures and/or outcomes into a single causality assessment. Under this approach, the above diagram changes from a standard Bradford Hill analysis that evaluates a single exposure and a single outcome to an analysis that includes multiple associations as set forth in figure 2 below:

Doing so allows a plaintiff's expert to pull from a broader (and potentially perceived as more advantageous or supportive) body of literature in support of a causation argument while still purportedly operating under a scientifically acceptable causality framework.
In the In re Acetaminophen litigation, several of the plaintiffs' experts conducted shared Bradford Hill analyses5 to reach the conclusion that in utero acetaminophen exposure was causally associated with autism spectrum disorder (ASD), attention-deficit/hyperactivity disorder (ADHD), and neurodevelopmental disorders (NDDs). Doing so implicated three separate associations in one Bradford Hill analysis. This approach is illustrated in figure 3 below:
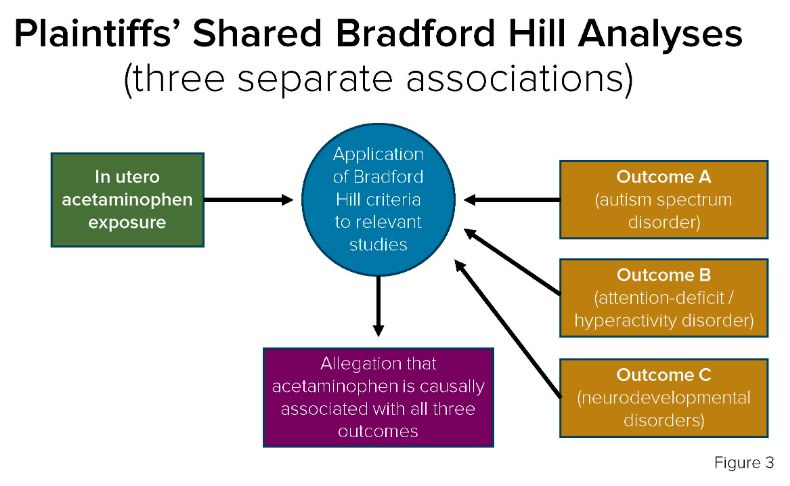 image
image
Intuitively, this practice raises questions about how one can interpret the results of a shared Bradford Hill analysis as compared to Bradford Hill analyses that separately evaluate each exposure and outcome pair. In a first-of-its-kind decision, the In re Acetaminophen court rejected the shared Bradford Hill analyses of the plaintiffs' experts.6
The Court's Exclusion of Plaintiffs' Shared Bradford Hill Analyses
To begin, the court observed that the plaintiffs' experts "reviewed the body of scientific literature regarding in utero exposure to acetaminophen and its possible impact on neurodevelopment."7 However, it noted that they did "not use[] that literature to tender discrete opinions regarding that exposure and the risk of ASD and the risk of ADHD," but instead "swe[pt] into their analyses (and conclusions) ASD, ADHD, and other neurodevelopmental disorders."8 The court identified several specific flaws with this approach that warranted exclusion of these shared Bradford Hill analyses.
First, the court noted that a shared Bradford Hill analysis "raises a question of relevance," as the litigation was "brought to obtain recovery on behalf of those who have been diagnosed with ASD or ADHD,"9 not anyone with a NDD. Yet the plaintiffs' experts incorporated scientific literature on other NDDs in their shared Bradford Hill analyses without explaining the "basis for doing so or confronting the complexities created by this conflation."10 The court, accordingly, found that "[i]t is not clear . . . that conducting a Bradford Hill analysis on multiple associations at once," as the plaintiffs' experts had done here, "is informative or reliable."11
Second, the court found that the shared Bradford Hill analyses "obscured limitations in the scientific literature."12 There "was no separate analysis of, for instance, the consistency among the findings . . . , the strengths of any association, or any other relevant Bradford Hill factor, before the results of [NDD] studies were combined with the conclusions they drew from the studies of ASD and ADHD."13 The court observed that if "the analysis were focused on a single disorder," as done in a standard Bradford Hill analysis, "the tensions among the studies and contradictory conclusions would be more evident and easily weighted by an epidemiologist."14As one example, the court noted that only one epidemiological study evaluating prenatal acetaminophen exposure and a diagnosis of ASD found an association, but the plaintiffs' "decision to lump all NDD studies together" had effectively "masked" that "weakness of the evidence."15 The court thus concluded that the shared Bradford Hill analyses of the plaintiffs' experts "permitted cherry-picking, allowed a results-driven analysis, and obscured the complexities, inconsistencies, and weaknesses in the underlying data."16
Third, the court rejected the plaintiffs' argument that a shared Bradford Hill analysis was appropriate because of symptom and diagnostic-criteria overlap. The court observed that "the diagnostic criteria of the two disorders are undeniably distinct," and that "[a] child may suffer from both disorders, from only one, or from neither."17 The plaintiffs' own expert psychiatrist also admitted that ASD and ADHD are " 'highly heterogeneous' both in terms of etiology and presentation."18 The court therefore concluded that the mere fact that "ASD and ADHD are both categorized as NDDs does not suffice" to justify the use of a shared Bradford Hill analysis.19 And the court similarly found that the fact that "there may be overlap in the underlying biology of some of the symptoms of the disorders, which may be relevant to a causal analysis" is not "the linchpin that allows the plaintiffs' Bradford Hill analyses to be admitted."20
Fourth, and lastly, the court found that a shared Bradford Hill analysis "is not a methodology that has been subjected to peer review and publication either generally or as applied to ASD or ADHD."21 While the plaintiffs' experts claimed that certain publications espoused the use of shared Bradford Hill analyses, the court noted that this literature related mainly to choices of design and analysis for future studies. It therefore concluded this literature did not "transform the observation that ASD and ADHD share some symptoms and are sometimes co-morbidities into carte blanche for conducting a single causal analysis for these two disorders."22
Key Takeaways from the Court's Decision
Several significant takeaways emerge from the court's decision that defense counsel should be aware of in terms of expert strategy for product liability matters:
- Plaintiff experts will need to overcome multiple—and potentially impossible—hurdles to establish that a shared Bradford Hill analysis is reliable and admissible under Federal Rule of Evidence 702. These hurdles include establishing that (a) any additional outcomes or exposures included in a shared analysis are relevant to claimed injuries, (b) significant overlap justifies the purported grouping and any complexities surrounding this issue are carefully addressed, (c) the shared Bradford Hill analysis is not improperly masking weaknesses with respect to any individual exposure and/or outcome analyses, and (d) the shared Bradford Hill analysis has been accepted and subjected to peer review outside the courtroom.
- Shared Bradford Hill analyses are novel methodologies that could be subject to Frye challenges in certain state-court jurisdictions. Although the court's decision was made under the Federal Rule of Evidence 702 framework, its observation that a shared Bradford Hill analysis "is not a methodology that has been subjected to peer review and publication either generally or as applied to ASD or ADHD"23 potentially broadens its impact. A reasonable argument can be made in certain Frye state-court jurisdictions that a shared Bradford Hill analysis—unlike a traditional Bradford Hill analysis—is a novel methodology, thus justifying a potential Frye inquiry into its admissibility.
- While the plaintiffs' shared Bradford Hill analysis involved a single exposure and multiple outcomes, the court's logic should also apply to a shared Bradford Hill analysis using multiple exposures and a single outcome. Even compounds within the same family can have significant differences in molecular structure and mechanisms of action. Thus, the court's reasoning that ASD and ADHD both being categorized as NDDs, along with a potential "overlap in the underlying biology of some of the symptoms," is not enough to justify a shared Bradford Hill analysis should also apply to shared analyses that incorporate multiple types of exposures.
- Practitioners should scrutinize similar causation methodologies, such as "weight of the evidence" approaches, for improperly combining multiple associations into a single combined analysis. The court's concern about the masking of weaknesses or inconsistencies in the scientific evidence for one outcome via the grouping of multiple outcomes into a shared analysis should be just as relevant for other causation methodologies, such as a "weight of the evidence" approach. If multiple exposures or outcomes are included in a single causation analysis—even outside the Bradford Hill criteria—a challenge to the admissibility of that causation analysis may be appropriate.
Footnotes
1 Hill AB. The Environment and Disease: Association or Causation?. Proc R Soc Med. 1965;58(5):295-300.
2 E.g., In re Mirena Ius Levonorgestrel-Related Prod. Liab. Litig. (No. II), 341 F. Supp. 3d 213, 268 (S.D.N.Y. 2018); In re Zantac (Ranitidine) Prod. Liab. Litig., 644 F. Supp. 3d 1075, 1253 (S.D. Fla. 2022).
3 Hill, supra note 1, at 299 ("None of my nine viewpoints can bring indisputable evidence for or against the cause-and-effect hypothesis and none can be required as a sine qua non.").
4 See, e.g., Nowinski C, et al. Applying the Bradford Hill Criteria for Causation to Repetitive Head Impacts and Chronic Traumatic Encephalopathy. Front Neurol. 2022;13:938163 ("The Bradford Hill criteria were created to help scientists transition from an observed association between two variables to a verdict of likely causation."); Schoultz M, Beattie, et al. Assessment of causal link between psychological factors and symptom exacerbation in inflammatory bowel disease: a systematic review utilising Bradford Hill criteria and meta-analysis of prospective cohort studies. Syst Rev. 2020;9(1):169 ("In a classic study, Bradford Hill proposed a set of criteria, namely the Bradford Hill criteria, to evaluate systematically whether there is a causal link between an exposure of interest and a health outcome."); Mammen G, Faulkner G. Physical activity and the prevention of depression: a systematic review of prospective studies. Am J Prev Med. 2013;45(5):649-657 (Bradford Hill criteria "judg[e] if a causal link exists between two variables").
5The court and the plaintiffs' experts also referred to these as "transdiagnostic" Bradford Hill analyses.
6 In re Acetaminophen – ASD-ADHD Prod. Liab. Litig., No. 22MC3043 (DLC), 2023 WL 8711617 (S.D.N.Y. Dec. 18, 2023).
7 Id. at *16.
8 Id.
9 Id.
10 Id. at *36.
11 Id. at *20.
12Id. at *21.
13 Id. at *42.
14 Id. at *21.
15 Id. at *28.
16Id. at *42.
17 Id. at *21.
18 Id. at *41.
19Id. at *21.
20 Id. at *42 (emphasis in original).
21 Id.
22 Id. at *44.
23Id. (emphasis added).
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.

