Holding: In Boehringer Ingelheim Pharms. Inc. v. Mylan Pharms. Inc., No. 2019-1172 (Fed. Cir. March 16, 2020) (non-precedential), the Federal Circuit upheld the district court's finding of invalidity of certain claims for obviousness and obviousness-type double patenting as not clearly erroneous.
Background: Boehringer sued several companies for infringement of U.S. Patents 8,853,156 ("the '156 patent), 9,173,859 ("the '859 patent), and 8,673,927 ("the '927 patent"), which relate to the treatment of type 2 diabetes mellitus with DPP-IV inhibitors such as linagliptin. The district court found the asserted claims of the '156 patent directed to ineligible subject matter, so trial proceeded only on the '859 and '927 patents.
The district court held claims 1, 14, 15, 20, and 21 of the '859 patent and claims 7, 9, 15, 17, 19, 25, and 26 of the '927 patent were invalid for obviousness-type double patenting in light of the claims of U.S. Patent No. 8,178,541 ("the '541 patent"). The court also held these claims invalid as obvious in view of U.S. Patent App. 2004/0097510 ("the '510 publication"), which is a published application from the same family as the '541 patent. The Federal Circuit affirmed.1
Claim 14 is illustrative for the '859 patent:
14. An oral tablet formulation comprising [linagliptin] in an amount of 2.5 mg or 5 mg optionally in combination with metformin, and a pharmaceutically acceptable carrier or diluent.
Claim 7, which depends from claim 1, is illustrative for the '927 patent:
1. A method of treating type II diabetes mellitus comprising administering to a patient in need thereof a pharmaceutically effective oral amount of [linagliptin], and a pharmaceutically effective amount of metformin, which is from 300 mg to 1000 mg once or twice a day, or delayed-release metformin in a dose of 500 mg to 1000 mg once or twice a day or 500 mg to 2000 mg once a day.
7. The method according to claim 1, wherein the [linagliptin] is administered in an oral dosage of 2.5 mg or 5 mg.
The '541 patent, the ODP reference, was filed June 20, 2008 and claimed benefit of U.S. provisional 60/409,312 filed Sept. 9, 2002.
Claim 44 of the '541 recites: A pharmaceutical composition comprising [linagliptin] and metformin.
Claim 45 of the '541 patent recites: A method of treating type II diabetes mellitus comprising administering to a patient in need thereof a pharmaceutically effective amount of [linagliptin] and metformin.
The '510 publication is a prior art published application of a Boehringer parent related to and having the same specification as the '541 patent. The specification of the '510 publication disclosed a genus of compounds, including linagliptin, and dose ranges for that genus of compounds:
The dosage required to achieve such an effect is expediently, by intravenous route, 1 to 100 mg, preferably 1 to 30 mg, and by oral route 1 to 1000 mg, preferably 1 to 100 mg, in each case 1 to 4 times a day.
'510 publication, ¶¶ 0003, 0245, 0300. Examples 4-6 of the '510 publication provided the only guidance in the prior art regarding preparation of oral tablets, and those disclosed tablets comprising 75 mg, 100 mg, or 150 mg "of active substance." ¶¶ 2899-2911.
District Court: The district court found that the overlapping range led to a presumption of obviousness and that a POSA would have obtained the claimed ranges with routine experimentation.
Timeline from the district court decision:
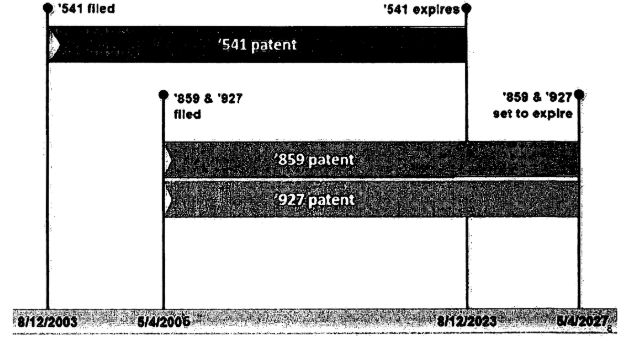
The district court concluded that the facts of this case were within Gilead, and Boehringer was seeking to "extend the '541 patent an additional four years through the '927 and '859 patent -
exactly the type of behavior that the obviousness-type double patenting doctrine seeks to prevent." Id. at *43.
The district court undertook a two-step obviousness-type double patenting inquiry. Step One: compare the claims. The district court found the only differences between the '541 reference patent claims and the asserted patent claims of the '859 and '927 patents were that the '541 patent claim 45 only recited "pharmaceutically effective amount of [linagliptin] and metformin" rather than specific dosages and the '541 patent claims 44 and 45 did not specify how the mode of administration. The asserted claims recited "2.5 mg or 5 mg" of linagliptin and were drawn to an oral tablet formulation or administration of an oral dosage of linagliptin.
Step Two: determine whether the asserted and reference claims are patentably distinct. The district court determined that the claims were not patentably distinct.
The Court noted that, although the '510 publication disclosed 7,000 compounds including linagliptin, it disclosed linagliptin as one in a list of 30 "most particularly preferred compounds" and as one of 6 compounds disclosed to have the lowest reported IC50 value (1 nM). The Court thus concluded that "a POSA would have been motivated to select linagliptin from the '510 publication, and begin a dose ranging study." District court decision, *47.
The court further found that a POSA would have been motivated to select the claimed 2.5 and 5 mg doses of linagliptin from the 1-100 mg range in the '510 publication. The court relied on a "presumption of obviousness" according to Tyco Healthcare Grp. LP v. Mut. Pharm. Co., Inc., 642 F.3d 1370, 1372-73 (Fed. Cir. 2011) since the claims fall within the prior art range. District court decision, *29. The court found that, alternatively, a POSA would have been motivated to conduct dose-ranging studies "starting at the low end of the 1 to 100 mg range, or starting at 75 mg, and dividing in half backwards, to optimize the linagliptin dosages" and that "routine experimentation" would have directed a POSA to the claimed 2.5 and 5 mg doses with a reasonable expectation of success. District court decision, *35-37.
In its obviousness analysis, the district court rejected Boehringer's objective evidence of unexpected results, long-felt but unmet need, and commercial success. For unexpected results, Boehringer argued that the unexpected result was linagliptin clearing through the body hepatically (i.e., through the liver) as opposed to renally (i.e., through the kidneys). Defendants argued that rather than an unexpected result, a POSA would have no expectation about how the body would clear linagliptin. The district court agreed, finding that "Boehringer offers no evidence which supports that the renal clearance of linagliptin was an unexpected result." Instead, the court found that Boehringer's expert admitted the a POSA would have had no expectation as to how linagliptin would be cleared. Moreover, the district court dismissed Boehringer's evidence on the low percentage of renal clearance was a difference in degree, not kind, as is necessary for unexpected results.
Boehringer argued that as of May 4, 2006, there was a long-felt but unmet need for a drug that did not require dose adjustment and was not contraindicated in patients with renal insufficiency. However, the only evidence submitted was an internal Boehringer document, which the district court held did not equate to a POSA recognizing a long-felt but unmet need. It also rejected post-priority date evidence as irrelevant. Finally, it found a lack of nexus between Boehringer's argument that renal impairment was the basis of the need when there is nothing about renal impairment in the claims or specification. In fact, "Boehringer admits that the most important aspect of the asserted claims is not linagliptin's effectiveness in treating patients with renal impairment, but the actual doses found in the asserted claims." Id. at 55.
The district court found that the evidence of commercial success was only "modest," and therefore insufficient to overcome a presumption of obviousness.
Federal Circuit: Judge Moore wrote for the panel of Moore, Dyk, and Hughes, affirming the routine experimentation rationale of the district court and declining to consider whether the district court's presumption determination was correct.
The Federal Circuit held that the district court did not clearly err in finding that a POSA would "have a reasonable expectation of arriving at the claimed 2.5 mg and 5 mg dosages" through routine experimentation. The Court also found no clear error in the district court's analysis of objective evidence. The Federal Circuit did not undertake its own double-patenting analysis.
Take-Away:
Careful consideration should be given to generic and specific disclosures of ranges, such as dosages, in different patents. This decision seems to put at risk later expiring dosage patents and may extend to later expiring patents that disclose specific properties and ranges that fall within an earlier generic disclosure.
Secondary considerations or objective evidence of nonobviousness should be documented by evidence that would have been known to a POSA and such evidence should be shown to have a nexus to the claims.
Endnotes
1The Federal Circuit also reversed the district court's judgment with respect to the subject matter eligibility of the '156 patent and remanded, but we are only discussing the obviousness-type double patenting and obviousness holdings in this article.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.




