President Trump and China vice-premier Liu He signed a preliminary Economic and Trade Agreement ("the Agreement") on January 15, 2020. In the press, it is referred to as "Phase 1." Chapter 1 of the Agreement includes provisions related to intellectual property, and among those provisions are some patent related provisions that should be welcomed by innovative pharmaceutical patent holders. According to Art. 8.3, the Agreement shall enter into force within 30 days of signature by both Parties or the date on which the Parties notify each other in writing of the completion of their respective applicable domestic procedures, whichever is sooner. It is probably safe to say that the 30-day deadline will come sooner, so the Agreement should enter into force on Feb. 14, 2020.
Patent Term Adjustment/Patent Term Extension
China agreed to extend patent terms for novel drugs "to compensate for unreasonable delays that occur in granting the patent or during pharmaceutical product marketing approvals." This should help protect pharmaceutical patent rights for both foreign and domestic innovative pharmaceutical companies. Providing additional days of patent term benefit drug patent owners because the last few years of a drug patent's life are usually the most lucrative, assuming the patent covers a product approved by a relevant regulatory authority. The longer such a patent is in force, the longer the owner can lawfully utilize the patent to prevent generic competitors from entering the market created by, for example, the approved product.
For example, Art. 1.12 roughly corresponds to 35 U.S.C. §154(b) (patent term adjustment or PTA) and 35 U.S.C. §156 (patent term extension or PTE) of the U.S. Patent Law. Article 1.12(a), focused on patent prosecution or administration relating to PTA, reads:
China, at the request of the patent owner, shall extend the term of a patent to compensate for unreasonable delays, not attributable to the applicant, that occur in granting the patent. For purposes of this provision, an unreasonable delay shall at least include a delay in the issuance of the patent of more than four years from the date of filing of the application in China, or three years after a request for examination of the application, whichever is later.
In principle, Art. 1.12(a) aligns China's patent system with U.S. 35 U.S.C. §154(b). It applies to all patents, not just those covering pharmaceutical products. Here, "unreasonable delay" is more than four years from the date of filing of the application in China, or three years after a request for examination of the application, whichever is later. In the U.S., PTA provides additional patent term to compensate for certain delays in prosecution, time beyond three years of application pendency, and delays from derivation proceedings, secrecy orders, and successful appeals. PTA also provides for subtraction of days awarded, where the delays are attributable to the patent applicant. A similar accounting is expected to appear in the proposed Fourth Amendments of Chinese Patent Law, which will likely become effective in 2020 if the U.S.-China trade negotiations go smoothly.
Art. 1.12(b), focused on the regulatory review process relating to PTE, provides:
With respect to patents covering a new pharmaceutical product that is approved for marketing in China and methods of making or using a new pharmaceutical product that is approved for marketing in China, China, at the request of the patent owner, shall make available an adjustment of the patent term or the term of the patent rights of a patent covering a new product, its approved method of use, or a method of making the product to compensate the patent owner for unreasonable curtailment of the effective patent term as a result of the marketing approval process related to the first commercial use of that product in China. Any such adjustment shall confer all of the exclusive rights, subject to the same limitations and exceptions, of the patent claims of the product, its method of use, or its method of manufacture in the originally issued patent as applicable to the approved product and the approved method of use of the product. China may limit such adjustments to no more than five years and may limit the resulting effective patent term to no more than 14 years from the date of marketing approval in China.
Here, "unreasonable curtailment" was not specifically defined as "unreasonable delay" was in Art. 1.12(a). It remains open how the China National Intellectual Property Administration (CNIPA) interprets "unreasonable curtailment" and how broadly they consider the "marketing approval process." But the compensation of patent term to the patent holder for delays in marketing approval is similar to that in the U.S. under 35 U.S.C. §156. In particular, China may grant the patentee up to a 5-year maximum extension, which 5-year maximum may be capped by 14 years from the date of marketing approval in China. That is the same as PTE in the U.S. In addition, PTE is available in China only for the marketing approval related to the first commercial use of the product in China, which is the same as PTE in the U.S.
In fact, prior to Phase 1 of the Agreement, China published such provisions on PTE in its proposed Fourth Amendments of China Patent Law on January 4, 2019.
Pre-Market Notification to Patent Holder
Another provision that should be welcomed by innovative pharmaceutical patent holders is one directed to creating a system of pre-market notification to the patent holder that a generic version is using the patented, approved product or method of use for seeking approval. See Art. 1.11. This system, often referred to as patent linkage, operates in the U.S. under the Hatch-Waxman Act. Until now, China had no equivalent; generic drugs could be approved in China and enter the Chinese market notwithstanding any Chinese patents they may be infringing. That left the patent holder in China with the necessity of suing for patent infringement in China on relevant Chinese patents.
In particular, Art. 1.11 (1) and (2) provide:
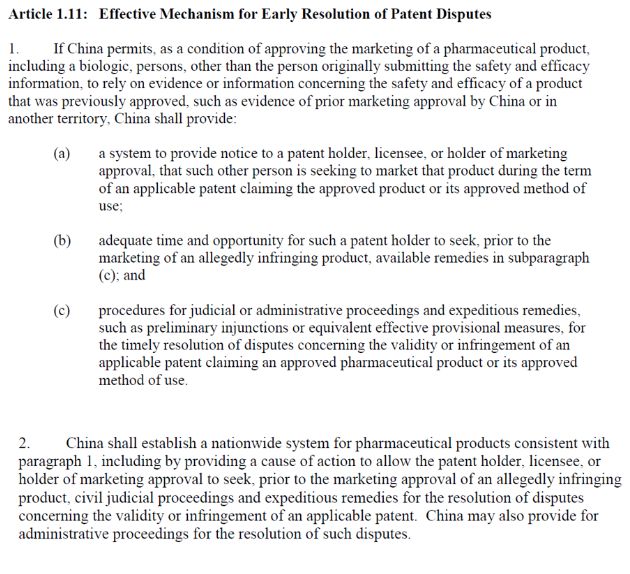
In view of those provisions, if China allows a third party, such as a generic manufacturer, to rely on safety and efficacy information submitted, for example, by a patent holder, then China shall provide a system of notice to the patent holder, "adequate time and opportunity" for the patent holder to seek available remedies before the generic product is marketed, and "procedures for judicial or administrative proceedings and expeditious remedies, such as preliminary injunctions or equivalent effective provisional measures, for the timely resolution of disputes concerning the validity or infringement of an applicable patent claiming an approved pharmaceutical product or its approved method of use."
In fact, paragraph (2) should sound familiar; it sounds very much like the U.S. procedure allowing a patent holder receiving a paragraph (IV) certification to institute civil judicial proceedings and seek remedies prior to marketing approval being granted. China may also provide administrative proceedings to resolve such disputes. Read together, (1) and (2) hold the promise of patent linkage in China, although Phase 1 of the Agreement does not expressly talk about a Chinese equivalent to the U.S. Orange Book. This appears to be a promising step forward in response to the repeated requests from innovative pharmaceutical companies regarding the establishment of the patent linkage system in China It is also a great addition to the proposed Fourth Amendments of Chinese Patent Law published on January 4, 2019, that, for some reason, did not mention patent linkage.
Post-Filing Supplemental Data
Another provision benefiting patent owners will allow pharmaceutical patent applicants to be able to rely on post-filing date, supplemental data like test results to show that the invention is sufficiently disclosed and has inventive step (i.e. non-obvious). See Art. 1.10. That has not been the case in China up until now, and such a development should further align the China and U.S. systems.
When Will Changes Become Evident?
Under Phase 1 of the Agreement, Art. 1.34 states that "Each Party shall determine the appropriate method of implementing the provisions of this Agreement within its own system and practice." Art. 1.35 requires China to provide a plan for implementation within 30 days of entry into force of this agreement. As noted above, "entry into force" will likely be Feb. 14, 2020.
But the devil is in the details. The value of these provisions will not be known until more details are known in terms of how both the broad PTA and PTE provisions will be implemented in China through laws, rules, and any interpretation issued by China's Supreme Court. The US PTA provisions, for example, are much more detailed in what types of delay will result in eligibility. For PTE, a number of questions remain. This Phase 1 of the Agreement does not provide any definitions for "pharmaceutical product" - will this cover human and animal drugs and biologics, medical devices and food or color additives? Will formulation patents be eligible for PTE? How is PTE to be calculated - when does the regulatory review period start, is there any reduction (partial credit) for activity in what is regarded as the "testing period" for a new drug in the U.S. Will already-issued patents be eligible for PTE? What if a patent is about to expire before the regulatory review process is complete? Will a patent be eligible for more than one PTE? Will testing conducted outside of China be eligible for consideration in the determination of PTE?
Whatever the final implementation looks like, Phase 1 of the Agreement is a significant first step toward a more user-friendly system for owners of Chinese drug patents. Over the coming months, hopefully there will some clarity to answer questions raised above and to indicate whether, in fact, the trade agreement effectively changes anything to the status quo.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.



