INTRODUCTION
On March 27, 2020, the US Food and Drug Administration issued a final guidance, entitled "Notifying FDA of a Permanent Discontinuance or Interruption in Manufacturing Under Section 506C of the FD&C Act" (506C Guidance).1 The 506C Guidance describes, and provides recommendations for fulfilling, the requirement for applicable drug and biologic applicants and manufacturers to notify FDA of a permanent discontinuance in the manufacture of certain drug or biological products or an interruption in the manufacture of such products that is likely to lead to a meaningful disruption in supply of those products in the United States.
While the 506C Guidance addresses section 506C of the Federal Food, Drug, and Cosmetic Act (FDCA) and its implementing regulations, which have collectively been in place since 2015, the ongoing Coronavirus Disease 2019 (COVID-19) pandemic prompted the release of this guidance. As a result of the COVID-19 pandemic, FDA has been closely monitoring the medical product supply chain and issued the 506C Guidance to assist applicable applicants and manufacturers in providing timely and informative notifications about changes in drug and biologic production to aid FDA in preventing or mitigating shortages of such products. On the same day FDA issued the 506C Guidance, the president signed the Coronavirus Aid, Relief, and Economic Security Act (CARES Act) into law. The CARES Act expanded the scope and requirements under section 506C of the FDCA and amended the FDCA to include similar reporting obligations for medical devices. A decision tree that summarizes the notification requirements under section 506C as amended by the CARES Act has been included at the end of this Advisory.
Background
Enacted on July 9, 2012, the Food and Drug Administration Safety and Innovation Act (FDASIA) amended section 506C of the FDCA to assist the FDA in addressing and mitigating drug shortages.2 As required by the FDASIA, FDA promulgated regulations implementing the requirements of section 506C on July 8, 2015.3
Under these requirements, applicable applicants and manufacturers must notify FDA of (i) a permanent discontinuance in the manufacture of certain drug and biological products or an interruption in the manufacture of such drug and biological products that is likely to lead to a meaningful disruption in the supply of these products in the United States and (ii) the reasons for such discontinuance or interruption. The standard for notification in the event of an interruption in the manufacture of blood or blood components intended for transfusion is where such interruption is likely to lead to a significant disruption in supply.4
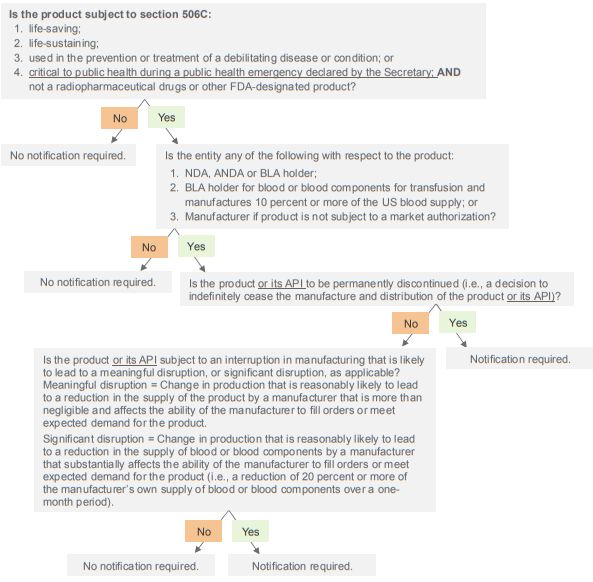
These notification requirements apply to drug and biological products that: (i) are either: (a) life-supporting; (b) lifesustaining; or (c) intended for use in the prevention or treatment of a debilitating disease or condition, including during emergency medical care or surgery; and (ii) are not radiopharmaceutical drugs or other FDA-designated products (Covered Products).5 As further discussed below, the CARES Act recently expanded these requirements to apply also to drug and biological products that are critical to public health during a public health emergency declared by the Secretary of Health and Human Services (Secretary) under section 319 of the Public Health Service Act (PHSA).6
506C Guidance
The 506C Guidance addresses the requirements of section 506C of the FDCA and its implementing regulations that existed prior to the enactment of the CARES Act, although many of the recommendations in the 506C Guidance are applicable to the CARES Act's additional reporting requirements. FDA implemented the 506C Guidance without prior public comment on the grounds that public participation was not feasible or appropriate due to the COVID-19 pandemic. While the 506C Guidance will remain in effect for the duration of the declared COVID-19 public health emergency, it will continue to reflect FDA's current thinking on this topic after it ends. As such, FDA intends to revise and replace the 506C Guidance within 60 days after the COVID-19 public health emergency terminates. Although already implemented, FDA is accepting public comment on the 506C Guidance.7
FDA considers the notification requirements under 506C of the FDCA to be critical in preventing or mitigating drug shortages. Without such early and informative notifications, FDA's ability to appropriately prevent, mitigate and respond to shortages may be limited. It is important to note that notifications regarding discontinuances or potential manufacturing issues required under other provisions of the FDCA do not substitute or supersede notifications required under section 506C.8
Responsible Persons
The persons responsible for providing required notifications for Covered Products to FDA under section 506C of the FDCA are as follows:
- Applicants with an approved new drug application (NDA) or abbreviated new drug application (ANDA);
- Applicants with an approved biologics license application (BLA), except for blood or blood components;
- Applicants with an approved BLA for blood or blood components for transfusion if the applicant manufactures 10 percent or more of the US blood supply; and
- Manufacturers of Covered Products marketed without an approved NDA or ANDA.9
With the exception of BLA holders for blood or blood components for transfusion, the notification requirement applies regardless of the applicant's or manufacturer's market share, availability of therapeutically equivalent products or amount of Covered Product in distribution. For Covered Products marketed under an NDA, ANDA or BLA, the market authorization holder is solely responsible for notifying FDA, regardless of whether it manufactures the Covered Product inhouse or through a contract manufacturer.10 Therefore, applicants should establish processes with any contract manufacturers or suppliers to ensure they can provide sufficient notification to FDA in a timely manner. If an applicant or manufacturer is unsure of whether the notification requirements apply, it can contact FDA.11
Notification Timing and Triggers
Generally, applicable applicants and manufacturers must notify FDA at least six months in advance of a permanent manufacturing discontinuance or an interruption in manufacturing of a Covered Product that is likely to lead to a meaningful disruption, or significant disruption, as applicable, in supply in the United States. Where this is not possible due to a reasonably unanticipated discontinuance or interruption, notification must be provided as soon as practicable and in any event no later than five business days after such discontinuance or interruption. FDA recommends that applicable applicants and manufacturers provide updates every two weeks, including the expected timeline for recovery, until the shortage has been resolved.12
A "permanent discontinuance" is interpreted by FDA to mean a decision by the applicant or manufacturer, as applicable, to cease indefinitely the manufacture and distribution of a Covered Product for any reason. FDA will assess the public health impact of reported discontinuances and may request further discussion with the applicant or manufacturer.13
A "meaningful disruption" occurs when there is a "change in production that is reasonably likely to lead to a reduction in the supply of a [Covered Product] by a manufacturer that is more than negligible and affects the ability of the manufacturer to fill orders or meet expected demand for its [Covered Product]."14 In determining whether a meaningful disruption is likely to occur, the applicant or manufacturer, as applicable, must base this assessment solely on its own capacity and supply and should not consider market demand for the Covered Product or other manufacturers' capacities.15 If an applicant or manufacturer is unsure whether a meaningful disruption is likely to occur, FDA recommends that a notification be submitted. Moreover, FDA recommends that a notification be submitted where an applicant or manufacturer is considering taking an action that may lead to a meaningful disruption, such as a transfer of ownership or holding production to investigate quality matters.16
For purposes of blood and blood components, a "significant disruption" occurs when there is a "change in production that is reasonably likely to lead to a reduction in the supply of blood or blood components by a manufacturer that substantially affects the ability of the manufacturer to fill orders or meet expected demand for its [Covered Product]."17 FDA will consider an interruption in manufacturing that leads to a reduction of 20 percent or more of an applicant's own supply of blood or blood components over a one-month period to constitute a significant disruption.18 Neither a "meaningful disruption" nor a "significant disruption" includes interruptions in manufacturing due to routine maintenance or similar matters or insignificant changes in manufacturing, so long as the manufacturer expects to resume operations in a short period of time.19
Even in the absence of a permanent discontinuance or meaningful or significant disruption, FDA requests that applicants and manufacturers notify the Agency whenever they are unable to meet Covered Product demand, such as when there is an unexpected spike in demand. While FDA acknowledges that this is not required, it encourages such reporting since it serves as a signal to the Agency that there may be a potential shortage and enables it to take appropriate action.20
Content of Notification
Section 506C of the FDCA and its implementing regulations require notifications submitted by applicable applicants and manufacturers to contain the following:
- Name of the Covered Product, its National Drug Code number or an acceptable alternative standard for identification and labeling for biological products;
- Name of the application holder for Covered Products authorized under an NDA, ANDA or BLA, or the name of the manufacturer for unapproved Covered Products;
- Whether the notification relates to a permanent discontinuance or an interruption in manufacturing;
- Description of the reason for the permanent discontinuance or interruption; and
- Estimated duration of the interruption in manufacturing.21
To further aid FDA in addressing Covered Product shortages, the Agency recommends that applicants and manufacturers use the following questions to elicit additional information for inclusion in notifications:
- Is the notification for an unavoidable or preventable supply disruption?
- What is the underlying reason or root cause leading to the notification? (A detailed and thorough explanation is requested to allow FDA to identify and use the most appropriate and effective mitigation tools.)
- What is the estimated date of onset of the interruption in manufacturing or supply disruption for the Covered Product? If a supply disruption has occurred, what is the estimated duration?
- If the notification is for a permanent discontinuance, what is the anticipated time frame for all existing Covered Product, whether on hand or in distribution, to be exhausted?
- What is the estimated market share of
the Covered Product? Is the entire market share affected by this
issue?
What is the estimated volume of historic monthly sales, usage or demand, as applicable, for the Covered Product? - Is the Covered Product manufactured on multiple lines or in multiple facilities?
- How much current inventory of Covered Product is at the applicant's/manufacturer's facility or warehouse?
- When will the last batch of finished Covered Product be released into distribution? Based on the current demand, how long is supply expected to last in the market without additional releases?
- Is there an emergency or reserve supply of the Covered Product? Is allocation of supply on hand or is reserve supply an option?
(x) Has public information been, or will be, provided to stakeholders and patients regarding the actual or potential shortage (e.g., Dear Healthcare Provider Letters, supply or shortage information posted on applicant/manufacturer website)?
(xi) Is there a proposal for FDA to review to expedite availability of the Covered Product? What can FDA do to help prevent or mitigate a supply disruption?22
Applicants and manufacturers do not need to answer all of these questions at the time of submitting an initial notification. Instead, FDA recommends providing an initial notification as soon as practicable and updating the notifications with additional information as it becomes available.23 As further discussed below, notifications must now also contain additional information required by the CARES Act.
Submitting Notifications to FDA
Notifications must be submitted to FDA in an electronic format that the Agency can process, review and archive.24 For Covered Products regulated by the Center for Drug Evaluation and Research (CDER), initial notifications should be submitted via: (i) e-mail at drugshortages@fda.hhs.gov; or (ii) the CDER Direct NextGen Portal at https://edm.fda.gov/wps/portal/. For Covered Products regulated by the Center for Biologics Evaluation and Research (CBER), initial notifications should be submitted via e-mail at cbershortage@fda.hhs.gov. Any and all updates to CBER or CDER should be submitted via e-mail.25
A separate initial notification should be submitted for each discontinuance or interruption, which can include all affected Covered Products. A separate initial notification should be provided for any newly affected Covered Product, such as a new strength, even if the underlying issue relates to a previously-reported interruption.26
Failing to Notify FDA
FDA will issue a noncompliance letter to an applicant or manufacturer that fails to provide notification of a permanent discontinuance or an interruption in manufacturing in compliance with section 506C of the FDCA or its implementing regulations.27 This includes instances where the applicant or manufacturer fails to notify FDA in a timely manner. An applicant or manufacturer must respond to a noncompliance letter no later than 30 calendar days after its issuance, detailing the reason for noncompliance and required information regarding the discontinuance or interruption. Unless FDA has determined that the noncompliance letter was issued in error or the applicant/manufacturer had a reasonable basis for not notifying FDA based on its response, FDA will post the noncompliance letter and any response on its website within 45 days of issuing the letter. FDA will redact such materials to protect trade secrets and confidential commercial information.28 FDA has issued noncompliance letters in the past and, given the Agency's focus in this area, we expect FDA will continue to take compliance action.29
Public Disclosure of Drug Shortages
FDA maintains public lists of drugs and biological products it has determined to be in shortage, which include, among other things, the reason for, and estimated duration of, the shortage based on information provided by the manufacturer as well as information provided to stakeholders and patients regarding the shortage. FDA adds a product only after it determines the product is in shortage. Therefore, a Covered Product that is the subject of a section 506C notification is not automatically added to such lists. FDA will remove a product from the "current shortage" section of the list once it considers a shortage to be resolved. FDA makes this determination based on an evaluation of the entire market, which includes assessing whether all backorders have been fulfilled and whether supply is meeting or exceeding demand. FDA does not include trade secrets or confidential commercial information on these lists and generally works with manufacturers to confirm the accuracy and appropriateness of information before it is publicly posted.30
CARES Act
As noted above, the CARES Act expands the reporting requirements set forth in section 506C of the FDCA. First, the notification requirements now also apply to drug and biological products that are critical to public health during a public health emergency declared by the Secretary under section 319 of the PHSA.31 Second, applicable applicants and manufacturers must also notify FDA where there is a permanent discontinuance in the manufacture of an active pharmaceutical ingredient (API) of a Covered Product or interruption in the manufacture of such API that is likely to lead to a meaningful disruption in the supply of the API.32 Third, notifications must also detail as applicable: (i) whether an API is a reason for, or risk factor in, the discontinuance or interruption, the source of the API, and any alternative sources for such API known by the manufacturer; and (ii) whether any associated device used for preparation or administration included in the drug is a reason for, or risk factor in, the discontinuance or disruption. It also emphasizes that the reasons for, and expected duration of, the discontinuance or disruption must be disclosed.33
In addition to these changes, the CARES Act requires each manufacturer of a Covered Product or of any API or associated medical device used for preparation or administration included in such Covered Product, to develop, maintain and implement a redundancy risk management plan. This risk management plan must address risks to the supply of each Covered Product for each establishment in which the Covered Product or its API is manufactured.34 Moreover, registered drug establishments must now submit annual reports on the amount of each drug that was manufactured, prepared, propagated, compounded, or processed by such establishment for commercial distribution. This information may also be required to be submitted at the time a public health emergency is declared by the Secretary.35 These new requirements, including the expanded section 506C reporting requirements, will go into effect 180 days after the enactment of the CARES Act.
The CARES Act also amended the FDCA to include reporting requirements for some medical devices similar to the requirements of section 506C. These requirements apply to devices: (i) that are critical to public health during a public health emergency, including those that are life-supporting, life-sustaining or intended for use during emergency medical procedures or surgery; or (ii) for which the Secretary determines that information on potential meaningful supply disruptions is needed during, or in advance of, a public health emergency. A manufacturer of such device must during, or in advance of, a public health emergency declared by the Secretary, notify the Secretary of a permanent discontinuance in its manufacture or an interruption of its manufacture that is likely to lead to a meaningful disruption in supply of the device in the US and the reasons for such discontinuance or interruption.36 Such notification must be made at least six months prior to the discontinuance or interruption or, where this is not possible, as soon as practicable.37
A permanent discontinuance does not include discontinuances resulting from an approved modification of the device. Similar to section 506C, a "meaningful disruption" means a "change in production that is reasonably likely to lead to a reduction in the supply of a device by a manufacturer that is more than negligible and affects the ability of the manufacturer to fill orders or meet expected demand for its product."38 It does not, however, include interruptions in manufacturing: (i) due to routine maintenance or similar matters or insignificant changes in manufacturing so long as the manufacturer expects to resume operations no later than six months; (ii) components or raw materials, so long as such interruptions do not result in a device shortage and the manufacturer expects to resume operations in a reasonable period of time; or (iii) that do not lead to a reduction in procedures or diagnostic tests associated with a device designed to perform more than one procedure or diagnostic test.39
Conclusion
The COVID-19 pandemic has emphasized the importance of mitigating potential medical product shortages. Even before this pandemic, however, addressing medical product shortages was a priority for FDA.40 As such, although not legally binding, the 506C Guidance serves as an important guide for manufacturers since it reflects FDA's current thinking in an area in which it has taken compliance action. We expect FDA to issue further guidance on the new CARES Act requirements. Medical products companies should ensure that they have procedures in place to comply with their new obligations.
Decision Tree for Evaluating Whether There Is an Obligation to Notify Under 506C Guidance and CARES Act41
Footnotes
1 FDA, Guidance for Industry, Notifying FDA of a Permanent Discontinuance or Interruption in Manufacturing Under Section 506C of the FD&C Act (Mar. 2020).
2 See Public Law 112-144; see also 21 USC § 356c.
3 See 21 CFR §§ 310.306, 314.81(b)(3)(iii), and 600.82.
4 See 21 USC § 356c; see also 21 CFR §§ 310.306, 314.81(b)(3)(iii), and 600.82.
5 Id.
6 See section 3112(a) of the CARES Act.
7 506C Guidance at 2.
8 Id. at 6.
9 Id. at 4; see also 21 USC § 356c; 21 CFR §§ 310.306, 314.81(b)(3)(iii), and 600.82.
10 506C Guidance at 7; see also 21 CFR §§ 314.81(b)(3)(iii)(a) and 600.82(a)(1).
11 506C Guidance at 7.
12 506C Guidance at 7; see also 21 USC § 356c(b); 21 CFR §§ 310.306(b), 314.81(b)(3)(iii)(b), and 600.82(b).
13 506C Guidance at 7.
14 506C Guidance at 3; see also 21 USC § 356c(h)(3); 21 CFR §§ 314.81(b)(3)(iii)(f), and 600.82(f).
15 506C Guidance at 8.
16 Id.
17 506C Guidance at 3; see also 21 CFR § 600.82(f).
18 506C Guidance at 3; see also 80 FR 39815, 38920–21.
19 506C Guidance at 3; see also 21 USC § 356c(h)(3); 21 CFR §§ 314.81(b)(3)(iii)(f), and 600.82(f).
20 506C Guidance at 8.
21 506C Guidance at 4–5; see also 21 USC § 356c(a); 21 CFR §§ 310.306(b), 314.81(b)(3)(iii)(c), and 600.82(c).
22 506C Guidance at 9–10.
23 Id. at 10.
24 506C Guidance at 10; see also 21 CFR §§ 310.306(b), 314.81(b)(3)(iii)(b), and 600.82(b)
25 506C Guidance at 10.
26 Id.
27 506C Guidance at 11.
28 Id.
29 FDA, Drug Shortages: Non-Compliance with Notification Requirement (last updated Jan. 17, 2020).
30 Id. at 11–12.
31 Section 3112(a)(1) of the CARES Act.
32 Section 3112(a)(2) of the CARES Act.
33 Id.
34 Section 3112(b) of the CARES Act.
35 Section 3112(e) of the CARES Act.
36 Section 3121 of the CARES Act.
37 Id.
38 Id.
39 Id
40 See FDA, Drug Shortages: Root Causes and Potential Solutions (Oct. 2019); see also FDA, Fiscal Year 2021 Justification of Estimates for Appropriations Committee.
41 Additions implemented by the CARES Act are underlined in the decision tree.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.


