The Nevada Department of Health and Human Services (DHHS) released today its 2019 Drug Transparency Report in connection with the state law requiring transparency related to essential diabetes drugs (EDD). Notably, the Report states that 695 drug national drug codes (NDCs) were included on the February 2019 EDD list published by Nevada. Of these, 155 drug NDCs, or 22.4%, experienced a "significant price increase" during the prior one and/or two year periods, whereas 536 NDCs, or 77.6%, did not experience such significant price increases. The most common rationales provided by manufacturers for these significant price increases included research and development investments, competitive value, changes in marketplace dynamics, product rebates provided, and infrastructure investments.
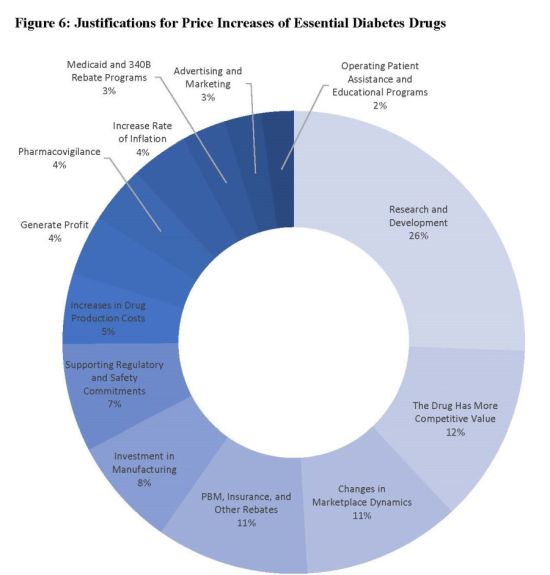
Drug manufacturers, especially those with products in the diabetes space, should review the entire report here as it also provides interesting information regarding PBM relationships, representative activities, and other key industry information.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.

