On November 17, 2023, the UK Competition and Markets Authority (CMA) published a Prioritisation Statement on combination therapies. The statement results from longstanding work and lobbying by the Association of the British Pharmaceutical Industry (ABPI) and its member companies, as well as the National Institute for Health and Care Excellence (NICE), and the UK National Health Service (NHS).
The Statement sets out the (very limited) circumstances in which the CMA will not open an investigation into competing pharmaceutical companies exchanging information and entering into commercial agreements on combination therapies for NHS patients.
Combination therapies and the CMA's interest
Modern medicine is progressively shifting away from the one drug-one target approach to treating cancers and other diseases. As new gene patterns and strains of disease-causing pathogens are discovered in patients, "magic bullet" monotherapies are slowly giving way to highly customised combination therapies.
A combination therapy comprises one or more "backbone" treatments and an "add-on" treatment, often developed by different companies. The backbone treatment is a drug that is already approved for use and reimbursement by the NHS and is available to NHS patients. The add-on treatment is added to an existing backbone treatment to create the combination therapy.
According to the ABPI, although combination treatments can often generate better health outcomes for patients, about 50% of them fail to obtain reimbursement approval from NICE. This is because the combined price of the component drugs is too high, and the combination treatment is not deemed "cost effective" under the UK access system.
Reimbursement challenges and the need for cooperation between drug manufacturers
NICE must issue a positive recommendation for the reimbursement of a combination therapy in order for the NHS to fund it and make it available to UK patients. For a combination therapy to obtain such a recommendation, the combined net price of its component drugs must be below the NICE's cost-effectiveness threshold. ABPI notes that this is very challenging to achieve in practice, partly because the confidential net prices of each drug component are independently negotiated and agreed between drug manufacturers and the NHS.
According to the ABPI, by the time a combination therapy is developed and approved by the MHRA, the price of the backbone treatment has typically already been negotiated, and it is often already close to the NICE's cost effectiveness threshold, leaving little, if any, scope to accommodate the additional cost of the add-on treatment. The association also noted that the backbone therapy supplier has a limited incentive to reduce the price of its component drug, as pricing regulations would require it to lower the drug price across all indications (even those unrelated to the combination therapy).
The Statement explains that, as a result of the above, the add-on treatment supplier is forced to reduce the price of its own component drug until the overall price of the combination therapy is below the 'cost effectiveness' threshold. In many cases, the extent of the price reduction is not regarded as commercially viable, and the combination therapy is abandoned.
The CMA has recognised that these challenges may result in reduced patient access to innovative treatments for serious diseases, and may also have a chilling effect on pharmaceutical R&D investment in the UK. In an effort to reduce those risks, the CMA has approved a negotiation framework developed by the ABPI. The framework allows drug manufacturers to negotiate a commercial agreement that would result in a combination therapy being supplied to the NHS at a 'cost effective' price, while minimising the risk that the negotiations and / or the resultant agreement would raise competition concerns.
The negotiation framework – DOs and DON'Ts
Under the framework, the suppliers of the component drugs of a combination therapy would negotiate and agree among themselves an amount per patient (defined in the Statement as the "contribution payment") to be paid by the backbone drug supplier to the add-on drug supplier. This contribution payment would compensate the add-on drug supplier for offering its component drug to the NHS at a price low enough that the combination therapy can meet the "cost effectiveness" threshold and be reimbursed. This process is summarised by the CMA using the diagram below:
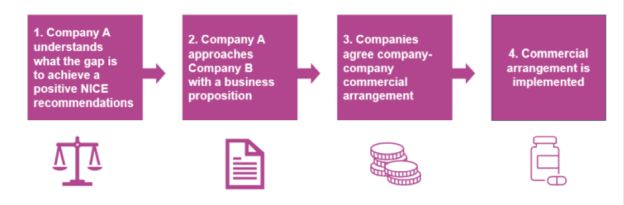
Source: CMA's Prioritisation Statement on combination therapies
The information exchanged between the suppliers has to be "limited to what is strictly necessary to reach the commercial agreement and to implement the mechanism for the contribution payment".
The Table below shows which information can and cannot be shared under the framework:
|
May share |
May not share |
|---|---|
| Proposed combination therapy and expected indication | Confidential net prices agreed with the NHS for the component drugs |
| NICE evaluation timelines and requirement for a response, for example confirming the backbone company is willing to engage during the evaluation process | Information allowing confidential net prices to be calculated through reverse-engineering |
| Clinical data / model assumptions pertaining only to the NICE assessment of the indication being evaluated | Pricing and discount information for products unrelated to the combination |
| Treatment pathway / line of therapy, including comparators / standard of care | Clinical data and model assumption unrelated to the assessment of the therapy being evaluated |
| Expected patient population numbers for the indication and assumed duration of treatment (including extrapolation analyses if applicable) | Development plans for competitive drugs unrelated to the combination therapy |
| The sum (£) the add-on company requires per patient to make its own (confidential) discount sufficient for a positive NICE evaluation and commercially viable. This includes details of what the 'per patient' amount is based on (i.e., per patient initiated on therapy, per cycle, patient weight, vial size, vial sharing, wastage etc.) | Commercialisation and product positioning plans for competitive drugs unrelated to the combination therapy |
| Proposed implementation of the agreement: data to be shared, source, and analysis of data; frequency of payments | |
| Proposed scoping meeting date, venue, agenda, terms of engagement if a discussion about the commercial agreement is required | |
| Proposed duration of agreement and any conditions for termination | |
| Public information, and any additional information that is reasonably necessary for the component suppliers to agree the contribution payments |
Additional requirements
Not all commercial agreements between drug manufacturers on combination therapies will be automatically clear of antitrust scrutiny. The following conditions must also be met:
- The drug manufacturers involved have independently agreed a confidential maximum net price for their respective drugs with the NHS (i.e. they have no ability to increase the price of the drug regardless of label expansions);
- Prescribing for the relevant combination therapy drugs is not primarily driven by price, but rather by clinical and cost effectiveness guidance published by NICE;
- The negotiations and exchanges of information between the component drug suppliers are carried out in line with the negotiation framework, in a good faith attempt to reach an agreement (even where one is not ultimately reached) with a view to making a combination therapy available to NHS patients;
- The terms of any agreement are directly related and
necessary for the calculation or operation of the contribution
payments, and they do not involve:
- Price fixing;
- Coordination for purposes other than obtaining reimbursement approval for a combination therapy;
- Amendment to the confidential net price paid by the NHS to the backbone medicine supplier;
- The contribution payment being conditional on any other conduct by the add-on drug supplier (e.g., exclusivity and non-compete restrictions).
- The manufacturers involved implement measures (e.g. confidentiality agreements) to ensure that the exchanged information is kept confidential and not disseminated more widely than necessary or used for any other purposes.
Takeaways
The CMA's Statement introduces helpful guidance for drug manufacturers working to bring highly innovative and effective combination therapies to UK NHS patients.
From a competition perspective, the approach is sensible in light of the existing UK regulatory requirements on drug pricing and prescription, which ensure that any information exchanged, or agreement reached, pursuant to the negotiation framework does not lead to higher prices to the NHS or poorer patient outcomes.
The scope of the Statement is, as might be expected, narrow. It assumes, in particular, that the backbone drug supplier has sufficient incentive to make contribution payments to the add-on drug supplier. This may not always be the case, particularly where the backbone drug supplier is unable to recoup the cost of the contribution payment (e.g., by treating the patients for longer thanks to the incremental health benefits of the combination therapy).
Absent commercial agreement between the drug suppliers on the contribution payment, suppliers of add-on treatments will continue to have limited leverage to satisfy the NICE's 'cost-effectiveness' threshold, beyond reducing the price of their own drug (potentially compromising their – and the combination therapy's - commercial viability).
The CMA acknowledged that there may be other means to make combination therapies available to NHS patients, and outside of the scope of the negotiation framework, parties will need to self-assess whether they are compliant with competition law. The CMA also confirmed that the Statement may be revisited in the future.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.



