Introduction
Inventive step of bio-related inventions is denied in cases where types, forms or the structure of chemical compounds in a bio and medical invention is easily acquired by a well-known technique and does not have an unexpected advantageous effect even if the invention does not specify a type, a form, or a structure of chemical compounds identical to that of the cited invention. Therefore, in order to determine inventive step in the invention, a patent applicant needs to assert that the form or structure cannot be easily obtained and/or that the invention does not result in an unexpected advantageous effect. The above two circumstances are described as follows:
- It is implied that a person skilled in the art cannot be easily achieved through conventional means
- An unexpected, advantageous effect is described as follows
- The present invention has a technical effect different from that mentioned in the cited invention and that effect cannot be conceived from a technical standard at the time when the patent application is filed.
- The present invention has a technical effect which is similar to that mentioned in the cited invention, but has a remarkably advantageous effect that cannot be conceived from the technical standard at the time when the patent application is filed.
The charts below illustrate what kinds of bio-related inventions are deemed as satisfying inventive step.
Fig. 1
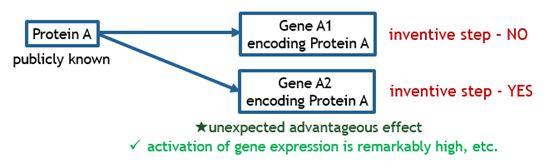
Fig.2
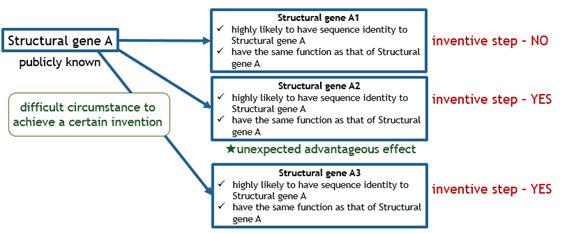
Fig. 3
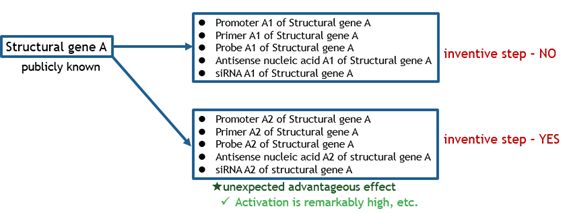
Fig. 4
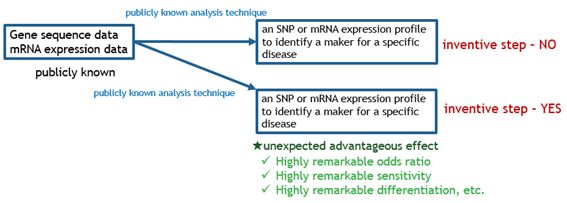
Fig. 5
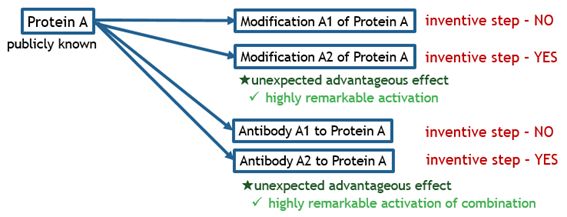
Fig. 6
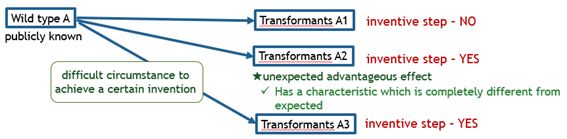
Fig. 7
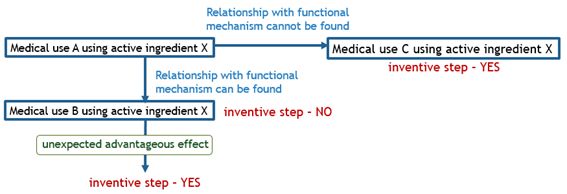
When determining inventive step, an invention is considered to have inventive step if advantageous effects of the invention remarkably exceeds beyond expectations in comparison with that of the cited invention. The following Supreme Court decision suggested a certain guideline for determining inventive step based on the assessment of unexpected advantageous effect of the present invention.
[Case Study of the Supreme Court Decision]
Title of invention: Topical ophthalmic formulations for treating allergic eye diseases
Japanese Patent Registration number: 3068858
Court Case number: 2018 (Gyo-hi) 69
Type: Trial against the JPO Appeal decision
Judgment date: August 27, 2019
Summary of the case:
This court case relates to whether histamine release inhibition of the present invention concerning an ophthalmic human conjunctival mast cell stabilizer, which is mentioned in the specification, has an advantageous effect.
In the second instance, the Intellectual Property High Court (IPHC) denied the advantageous effect of the claimed compound in the present invention given that there exists multiple agents with histamine release inhibition effects with the same amount as that of the present invention in other chemical compounds which have different structure from that of the claimed compound.
The Supreme Court decided that the IPHC decision was illicit in that the IPHC examined the advantageous effect only judging from the fact that multiple chemical compounds with different structure from the claimed chemical compounds were publicly known at the timing of priority date.
Finding a cited invention
Secondly, as specifications and research papers of bio medical inventions describe various feasibilities of claimed inventions, the issue will be whether the claimed invention has enough grounds to deny inventive step of the description mentioned in its prior arts. Bio and pharmaceutical inventions do not designate "cited inventions" in the following conditions. If the reason for rejection indicates lack of inventive step due to the following statements, a patent applicant is allowed to raise an objection that the cited invention cannot be used.
- It is obvious that production or acquisition of chemical compounds, etc. related to medical inventions is feasible based on technical common knowledge at the timing of filing or in accordance with description of publications. Therefore, in cases where the description is not mentioned in the publications, a bio medical invention may not be deemed stated in the publications.
- It is obvious that chemical compounds can be used for medical purpose based on technical common knowledge at the timing of filing or in accordance with description of publications. Therefore, if the description is not mentioned in the publications, a bio medical invention is may not be deemed stated.
[Example] If medical purposes are merely listed without any supportive evidence, in other words, even if the medical purpose is described in the cited invention but has no evidence to support it, there would be room to raise an objection against the JPO's reasons for rejection.
Late submission of experimental data
Since bio-medical inventions require time to generate certain results from an experiment, a patent applicant sometimes cannot prepare enough experimental data at the time of filing. It raises the question on whether late submission of experimental data after the filing may be referred to examining inventive step. In Japan, late submission of experimental data can be taken into consideration if the data can be inferred from the description of the specification. In the following circumstances, the Examiner considers advantageous effect(s) in comparison with the cited invention claiming in the opinions, etc.
- The advantageous effect is described in the specification.
- Although the advantageous effect is not described in the specification, the person in the skilled art can conceive its effect from the description of the specification or the drawings.
In other words, if an advantageous effect is not described in a specification and cannot be conceived from description of the specification or its drawings, experimental data which is submitted after its filing is not inferred in examining inventive step. Note that late submitted experimental data, which may be formally described in a specification, will most likely not be referenced to if its advantageous effect cannot be conceived in consideration of the experimental results suggested in the working examples and common technical knowledge.
Different practice in Japan, the U.S., and Europe
Japanese practice on assessing unexpected advantageous effect in terms of examining inventive step is similar to the US practice to some extent. The significant difference between the two is that, in US practice, the effect serves as supportive evidence in maintaining non-obviousness even after the effect is found after its filing overall. This is not present in Japan, however.
Under the Japanese practice on examining inventive step, "technical problems" to be solved by the invention focuses on the problems mentioned in the description of the invention. In Europe, "problems" is to be determined in light of the closest prior arts to the invention. As shown the above, each country has a different approach in assessing an advantageous effect. Patent applicants and patentees need to carefully check the practice of each country.
Summary
As described the above, it is necessary for a patent applicant to claim a specific tough circumstance or/and unexpected advantageous effect in order to maintain inventive step in a bio and medical invention. Then, when the applicant raises an objection against the JPO's reason for rejection regarding lack of inventive step, the applicant has two options which are to refer to a cited invention or to consider experimental data after the filing. Some recent court cases handled by the Supreme Court of Japan have determined unexpected advantageous effects on examining inventive step. The Supreme Court decisions would affect to the assessment of inventive step at the IPHC and JPO. Patent applicants and patentees should be aware of changing practice on examining inventive step.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.

