A. Introduction
Assisted reproductive technology is not a new concept to the world, especially to India. An interesting paper published by the Indian Journal of Endocrinology and Metabolism analyses our own ancient epic Mahabharata through the prism of reproductive endocrinology. The paper refers to the story of Gandhari and the lump of mass she gave birth to. As the legend goes, the "fetus" was divided into a hundred pieces and incubated. Finally, 101 children were born, one by one. This narrative is strongly reminiscent of in vitro fertilization (IVF), with the multiple pregnancies that commonly occur with it1.
In the recent times, the world witnessed successful birth of the world's first test tube baby in 1978, and thereafter we have seen advancement and acceptance of the assisted reproductive technology procedures. Such advancement was also followed by the necessity for legislation regulating the practice of assisted reproductive technology procedures. The same was recognized by the Indian Council of Medical Research (ICMR) as well by passing National Guidelines for Accreditation, Supervision & Regulation of ART Clinics in India in 20052. After more than a decade of issuing such guidelines, finally an act regulating the assisted reproductive technology was passed in 2021 by our legislature. The Assisted Reproductive Technology (Regulation) Act, 20213 ("Act") was passed on 18th December, 2021 and came into force on 25th January 2022 with the purpose of regulating and supervising the assisted reproductive technology (ART) clinics and banks and to ensure safe and ethical practices of ART in India. On 7th June, 2022 Assisted Reproductive Technology (Regulation) Rules, 20224 were passed ("Rules").
B. ART CLINICS
Section 2 (c) of the Act defines ART clinics as follow,
"assisted reproductive technology clinic" means any premises equipped with requisite facilities and medical practitioners registered with the National Medical Commission for carrying out the procedures related to the assisted reproductive technology"
Rule 3 (1) of the Rules provide for two levels of ART clinics,

C. Registration of ART Clinics:
The Act makes registration mandatory for ART clinics. The application for registration is provided to be made within 60 days of establishment of National Registry.

The registration certificate shall initially be granted for a period of Five years post which the same needs to be renewed for another period of Five years.
D. Duties of ART Clinics:
The Act and the Rules have provided for the duties and responsibilities of the ART clinics in detail through Chapter IV. Among various duties, broad duties are provided under Section 21
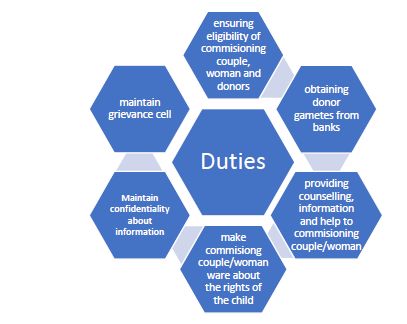
Written Consent and Restriction on use
There is restriction on ART clinics on performing any procedure without written informed consent of the Parties seeking assisted reproductive technology. ART clinics also cannot cryo-preserve any human embryos or gamete, without specific instructions and consent in writing from all the parties and use any human reproductive material, except in accordance with the provisions of the Act.
Accurate records
ART clinics have an obligation of maintaining accurate records at least for a period of Ten years, post which such records shall be transferred to the database of National Registry (as established under the Act). These records are also to be made available for inspection for National/State Board and National Registry. If any criminal or other proceedings are instituted against any clinics or banks, the records and all other documents of such clinics and banks shall be preserved till the final disposal of such proceedings.
Other Duties and Prohibitions
The Act provide for detailed responsibilities of the ART clinics with respect to use of human gametes and embryo and Preimplantation Genetic Diagnosis. One of the most important provisions of the Act is prohibition on the clinics to offer to provide a couple or woman with a child of a pre-determined sex and to determine the sex of the child to be born through the process of assisted reproductive technology.
The Rules have also provided for specific staff requirements and qualifications, list of equipment, grievance redressal form and other duties of ART clinics.
E. Offences and Penalties:


For any offence committed by an ART clinic, the executive head of such clinic is deemed to be guilty of the offence as per the Act under Section 37 (1) and shall be liable to be proceeded against and punished accordingly unless he proves that the offence was committed without his knowledge or that he had exercised all due diligence to prevent the commission of such offence.
We believe that the ART Clinics need to have SOPs to meet compliance requirements of the Act.
F. Impact
The impact of this Act can be assessed through a recent judgement passed by the High Court of Kerala in Rakhi Bose Vs. Union of India5, on 21st day of June, 2022. In this Case, the Petitioners, a married couple had undergone oocyte retrieval procedure in the year 2014 and the embryos were then preserved at the 7th respondent hospital. In 2016, the couple had stopped taking infertility treatment following Chief Consultant's advice.
However, the couple re-commenced their treatment at the 8th respondent hospital. As advised by the Doctors at the 8th respondent, the couple requested for the 7th respondent's permission to transfer the frozen embryos to the 8th respondent. During this period, the Act came into force, bringing in prohibition with respect to transfer of embryos. Accordingly, the 7th respondent hospital replied to the couple stating the same.
Hence the couple filed this writ petition for an order or direction from the Court, commanding the respondents 1 to 3 to constitute National Board as contemplated in Sec. 29 of the Act and Surrogacy Act, 2021 or ad hoc National Board to enable it to grant permission for the couple to transfer their own frozen embryo kept in 7th respondent hospital to 8th respondent hospital, for their own use and to grant urgently such permission as applied for. Considering the facts of the case the provisions of the Act, the Court held that, "...The primary objective of the Act is the regulation and supervision of the assisted reproductive technology clinics and banks, by preventing misuse and ensuring safe and ethical practice of assisted reproductive technology services. The Act is not intended to create difficulties for persons opting assisted reproductive procedure." The Court had to intervene by issuing appropriate orders, allowing the couple to have the embryo transferred.
G. Conclusion
The recent news of Illegal oocyte sale at Tamil Nadu6 where a sixteen-year-old girl complained to the police that she was forced to sell her oocytes to private hospitals using a forged Aadhaar card, has nothing but emphasized the need of the hour of a strong legislation.
Considering the mushrooming of ART clinics and increasing number of couples opting for the assisted reproductive technologies (ART), the Legislature has tried to address the issue through the Act, but it remains to be seen whether this Act can stand the test of constitutional validity imposed on it through a petition filed before the Delhi High Court7.
The Act along with the Surrogacy (Regulation) Act, 2021 is currently challenged in the Delhi High Court of being discriminatory and ultra vires Article 14 and 21 of the Constitution of India, alleging that it covers the right of only commissioning couple and women (as defined in the Act) thereby excluding from its purview single men, LGBTQ community, couples in live-in relationship and secondary infertility.
* The article reflects the general work of the authors and the views expressed are personal. No reader should act on any statement contained herein without seeking detailed professional advice.
Footnotes
1 The Mahabharata and reproductive endocrinology - PMC (nih.gov) - 2016 Indian Journal of Endocrinology and Metabolism | Published by Wolters Kluwer - Medknow
2 https://main.icmr.nic.in/sites/default/files/art/ART_Pdf.pdf
3 https://egazette.nic.in/WriteReadData/2021/232025.pdf
4 https://egazette.nic.in/WriteReadData/2022/236395.pdf
5 WP(C) NO. 1 9184 OF 2022
7 Writ Petition 8448 of 2022
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.

