The federal government has amended the Food and Drug Regulations (FDR)1 and the Safe Food for Canadians Regulations (SFCR)2 to update the requirements for labelling prepackaged food products (Modernized Food Labelling Framework). Health Canada has also amended the FDR3 to introduce a new requirement for front-of-package nutrition symbol labelling (FOP Labelling) for select prepackaged products containing nutrients of public health concern (saturated fat, sugars and sodium), and to update the requirements for certain nutrient content claims.
What you need to know
- Modernized Food Labelling Framework. Amendments to the FDR and SFCR introduce updated labelling requirements relating to standard container sizes, ingredient names, test market foods, and food commodity-specific labelling requirements. The amendments to the FDR and SFCR are part of the Canadian Food Inspection Agency's initiative to modernize the food labelling framework to reflect current market realities including consumer demand and industry practices.
- New FOP Labelling requirement and other updates for prepackaged food products. These amendments to the FDR introduce a requirement for FOP Labelling for most prepackaged products containing nutrients of public health concern (saturated fat, sugars and sodium) at or above specified thresholds. The change is designed to help consumers identify foods that are high in these nutrients and help reduce associated risks from excess consumption. The amendments to the FDR also update requirements for nutrient content claims, vitamin D fortification, and labelling of high-intensity sweeteners, and reflect the prohibition of partially hydrogenated oils (PHOs).
- In force dates. The amendments introducing the Modernized Food Labelling Framework came into force on June 21, 2022. The amendments introducing FOP Labelling and related amendments to the FDR came into force on July 20, 2022, with a transition period that ends on December 31, 2025.
Changes introduced by the Modernized Food Labelling Framework
Repeal of standard container size requirements
Prescribed standard container sizes for a number of prepackaged food products (e.g., certain fresh fruits and vegetables) have been repealed from the SFCR, and the remaining list of sizes has been incorporated by reference in the standard container sizes document.
Increased flexibility for ingredient names
The list of mandatory and optional common names (which the FDR previously required or permitted for identifying certain foods and classes of foods, e.g., "vegetable oil" and "seasonings") was repealed from the FDR and incorporated by reference in the common names for ingredients and components document. Consequential amendments were also made to the Cannabis Regulations4 to reflect these amendments. These amendments were made in response to consumer and industry concerns regarding the clarity and flexibility of common names, and to increase regulatory alignment with major trading partners to expand market access5.
"Test market foods" defined
A definition for "test market food" was added to the SFCR to clarify the intent of a Test Market Authorization (TMA), by limiting the application of TMAs to foods that were not previously sold in Canada in the current form and that differ substantially from any other food sold in Canada with respect to composition, function, condition or packaging form. The amendment is intended to limit food businesses from seeking TMAs as a means to obtain exemptions from regulatory requirements for food products, such as standard container sizes6. Existing TMAs will continue to apply until their expiry date.
SFCR and FDR commodity-specific labelling requirements streamlined
Several food commodity-specific labelling requirements were streamlined by either repealing the commodity-specific labelling requirement or introducing a single requirement that applies to all prepackaged food products. For example, several previously existing labelling requirements were replaced by a new provision in the FDR that requires the packaging of certain foods to include words that distinguish them from other similar foods (e.g., distinguishing tuna packed "in brine" from tuna packed "in oil").
Additionally, certain food descriptors in the SFCR (such as "soft", "semi-soft", "firm", and "ripened" for cheese) are now incorporated by reference in the descriptive words, expressions and identification names document instead of in the SFCR itself. Other food commodity-specific labelling requirements were not affected by the amendments (e.g., those required for health and safety reasons, to protect consumers from false and misleading information, or for trade reasons).
SFCR licensing requirements clarified
Amendments to the SFCR were made to clarify that restaurants and other similar enterprises with cross-provincial border operations are not subject to the SFCR licensing requirements.
Implementation
Amendments to the FDR and SFCR came into force on June 21, 2022. There is no transition period for implementation of these amendments, with the exception that foods that are currently being sold under a TMA may continue to be sold until the expiry of the TMA.
Changes introduced by the new FOP Labelling requirements and other labelling changes
New FOP Labelling requirements
The new FOP Labelling requires that a nutrition symbol be displayed on the front of packaging of a prepackaged food product that contains or exceeds a certain threshold of saturated fat, sugars or sodium. The amendments to the FDR have introduced prescribed requirements for the placement and format of the FOP Labelling. One example of such a format is set out below.
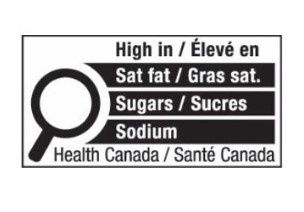
Parallel to the amendments to the FDR, Health Canada published a guide on front-of-package nutrition symbol labelling7 that is intended to assist stakeholders in the food industry understand the core requirements of the FOP Labelling.
Certain foods or types of foods are exempted or prohibited from the FOP Labelling requirement. The amendments to the FDR also introduced restrictions on nutrient content and health-related claims when the label of a prepackaged food product contains FOP Labelling. For example, an "unsweetened" claim is prohibited on the principal display panel of a prepackaged food product when a product contains FOP Labelling for "high in sugars". Additionally, it is prohibited to use representations on packaging that are likely to be mistaken for FOP Labelling, or to advertise or sell such products.
Updates to nutrient content claims
The table of nutrient content claims (which prescribed nutrient content claims such as "low in sodium" and "no added sugar" and provided the conditions for use of such claims) was repealed. In its place, nutrient content claim requirements were incorporated by reference via a new table of permitted nutrient content statements and claims, to permit these claims to be more easily updated in response to new evidence and related nutrition labelling policies8. In parallel, Health Canada published a notice of modification outlining changes to the conditions for certain nutrient content claims and the introduction of a new "low in sugars" nutrient content claim9.
The FDR were also further amended to permit representations regarding alcoholic content for beverages with less than 0.5% alcohol and to update references to food "intended solely for children under two years of age" to reflect new age categories.
Other changes for prepackaged foods
Other changes introduced by the amendments to the FDR include the following:
- increasing the amount of vitamin D required in cow's milk and margarine, and permitted in goat's milk;
- modifying the formatting and placement requirements for aspartame declarations for products containing aspartame;
- repealing certain high-intensity sweetener labelling requirements10; and
- removing references in the FDR to PHOs due to Health Canada's previous decision to prohibit their use in foods via the list of contaminants and other adulterating substances in foods.
Transition and implementation
The FOP Labelling requirements, updates to nutrient content
claims and other changes for prepackaged foods discussed above came
into force on July 20, 2022. However, manufacturers will have a
transition period ending December 31, 2025 to comply with new
requirements. Products imported, manufactured in Canada or packaged
at retail before this transition period ends can remain in the
warehouse and continue to be sold in stores11.
This bulletin was written with assistance from Luke Jeagal and Nushrah Amod.
FOOTNOTES
1. Regulations Amending the Food and Drug Regulations, SOR/2022-143, available online at: https://gazette.gc.ca/rp-pr/p2/2022/2022-07-06/html/sor-dors143-eng.html.
2. Regulations Amending the Safe Food for Canadians Regulations, SOR/2022-144, available online at: https://www.gazette.gc.ca/rp-pr/p2/2022/2022-07-06/html/sor-dors144-eng.html.
3. Regulations Amending the Food and Drug Regulations (Nutrition Symbols, Other Labelling Provisions, Vitamin D and Hydrogenated Fats or Oils), SOR/2022-168, available online at: https://canadagazette.gc.ca/rp-pr/p2/2022/2022-07-20/html/sor-dors168-eng.html.
4. Regulations Amending the Cannabis Regulations (Listing Ingredients for Edible Cannabis), SOR/2022-145, available online at: https://www.gazette.gc.ca/rp-pr/p2/2022/2022-07-06/html/sor-dors145-eng.html.
5. Regulatory Impact Analysis Statement, Regulations Amending the Food and Drug Regulations, SOR/2022-143 (6 July 2022) C Gaz II, vol 156, no 14, online: https://gazette.gc.ca/rp-pr/p2/2022/2022-07-06/html/sor-dors143-eng.html.
6. Ibid.
7. Guidance document: Front-of-package nutrition symbol labelling guide for industry, available online at: https://www.canada.ca/en/health-canada/services/food-nutrition/legislation-guidelines/guidance-documents/front-package-nutrition-symbol-labelling-industry.html.
8. Regulatory Impact Analysis Statement, Regulations Amending the Food and Drug Regulations (Nutrition Symbols, Other Labelling Provisions, Vitamin D and Hydrogenated Fats or Oils), SOR/2022-168 (20 July 2022) C Gaz II, vol 156, no 15, online: https://canadagazette.gc.ca/rp-pr/p2/2022/2022-07-20/html/sor-dors168-eng.html.
9. Notice of modification: Incorporating by reference the "Nutrition labelling – Table of permitted nutrient content statements and claims", available online at: https://www.canada.ca/en/health-canada/services/food-nutrition/public-involvement-partnerships/modification-table-permitted-nutrient-content-statements-claims/information-document.html.
10. The high-intensity sweetener labelling requirements relate to additional, non-health based, principal display panel and quantitative declaration labelling requirements for foods containing aspartame, sucralose, acesulfame-potassium and neotame.
11. The transition period does not apply to the amendments that repeal certain high-intensity sweetener labelling requirements and remove references in the FDR to PHOs.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.



