On September 5, 2018, the European Chemicals Agency ("ECHA") launched a public consultation to include 18 new substances in Annex XIV REACH. REACH, the acronym for the Registration, Evaluation, Authorisation and Restriction of Chemicals, is an EU regulation that addresses the production and use of chemical substances, as well as their potential impacts on human health and the environment. ECHA's consultation is open for comments until December 5, 2018.
The substances under consideration for authorization include the following:
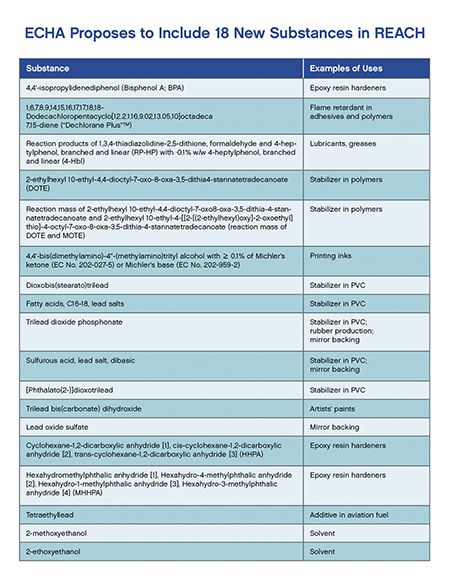
Simultaneously, the European Commission will be investigating the socioeconomic impact of inclusion of the substances into Annex XIV. After the public consultation and the Member State Committee's opinion, the Commission will make a final decision on insertion into Annex XIV.
By way of background, once a substance is listed in Annex XIV, it may no longer be used in the European Union unless a company-specific "Authorization" has been granted, which is a lengthy and expensive procedure (Title VII of REACH) involving ECHA, the Commission, and EU Member States. Moreover, each Authorization is subject to regular review, repeating the lengthy and expensive procedure. Either manufacturers or importers of the substance or the actual users can apply for Authorization.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.


