Using software and drugs together to treat a patient may create "a combination product." For example, in early 2019 Pear Therapeutics launched reSET-O, an app for treating addictions. Pear claims the app can provide interactive therapy sessions, including cognitive behavioral therapy, fluency training, exercises, and contingency management for those taking buprenorphine to help recover from opiate addiction. The app, available by prescription only, was shown when combined with buprenorphine in clinical trials to be better than buprenorphine alone in helping people overcome addiction.
A combination product is composed of at least two components reviewed by different centers at the FDA. Either a drug or biologics product used with a device constitutes a combination product, or a drug and a biologics together can be considered a combination product. The 2016 CURES ACT provides special protection for such combination products, including access to patent infringement provisions of 35 U.S.C. § 271 (e)(2) and (e)(4). These provisions offer additional avenues for both the software developers and the pharma companies to benefit from their respective contributions not available to either alone.
The FDA treats AI/machine learning (ML) that is in software used by the patient as a medical device (SaMD). The benefits of such a SaMD reside in its ability to learn from real-world use and experience in order to improve its performance. The FDA also recognizes that AI/ML-based SaMDs exist on a spectrum categorized by risk to patients and on a spectrum from locked to continuously learning. "Locked" algorithms are those that provide the same result each time the same input is provided. As such, a locked algorithm applies a fixed function (e.g., a static look-up table, decision tree, or complex classifier) to a set of inputs. These algorithms may use manual processes for updates and validation. In contrast to a locked algorithm, an adaptive algorithm (i.e., a continuous learning algorithm) changes its behavior using a defined learning process. The algorithm adaptation or changes are implemented periodically based on the inputs. The output may be different before and after the changes are implemented, reflecting the learning process. These algorithm changes are typically implemented and validated through a well-defined and possibly automated process that aims at improving performance based on analysis of new or additional data.
The Food, Drug & Cosmetics Act requires FDA approval of software that will affect the patient. The degree of review depends on the risk associated with using the SaMD in the clinical setting. The FDA provides the following chart to determine the class the SaMD falls into:
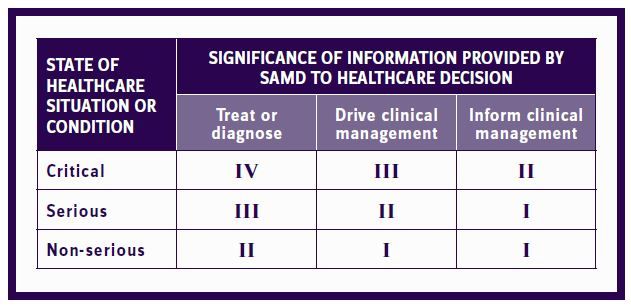
Figure 1: SaMD The International Medical Device Regulators Forum risk categorization
Category I generally requires only a simple premarket notification. Categories II to IV will most often require clinical trials to demonstrate both safety and efficacy. If clinical trials are required, then any patent on the software may be entitled to a patent terms extension (PTE) to compensate for the time lost in the clinical testing and regulatory review. The extension may be for up to five years, but it may not extend the patent life longer than 14 years from the FDA approval date. With software, the availability of PTEs is mostly theoretical since FDA approval is usually much quicker for SaMD than for drugs. The availability of a PTE may become important if the software is tied to a drug undergoing FDA review and will be approved only upon the approval of the drug. The importance of the combination product is the provision that the software will be treated as a drug for the purposes of litigation. Anyone seeking FDA approval to sell a generic equivalent of the approved combination must provide notice of filing the ANDA application to the owners of both the patents on the drug and the software and the NDA holder (if different). This allows the patentees and the NDA holder to initiate litigation within 45 days of the notice and obtain an automatic 30-month stay of FDA approval of the generic application. There are exceptions where the label lists different indications (diseases) for treatment by the drug that are not labeled for use with the software. In this case the generic may "carve out" from its label the indication and the associated software from the label and avoid the notice requirement. This is called a "skinny viii" carve out. A carve out is not possible where the software applies to all the indications. This can be achieved when the software impacts the dose taken or the dosing schedule.
Usually the expiration date of the software patent will be well after that of the drug since the software will probably have been developed as part of the clinical trial period, which usually begins years after the drug patent is filed. Often, it may be even after the drug is approved that the companion software will be developed. The patent on the software can extend the drug's exclusive period beyond its patent protection up to the expiration of the patent on the software.
The patent on the software allows pharma to obtain additional protection for its drug if the drug is labeled for use with the software as the generic must also be labeled identically. If the software patent is found infringed, then the resulting injunction will preclude the FDA from approving the generic application. Even if the use of the software is optional, the generic equivalent still cannot be approved even for use without the software as its label must be identical to the pharma's label and include the use with the software. This preserves the drug company's exclusivity on the drug until the software patent expires, which will normally be the last patent to expire. The advantage to the software company is that its agreement with the drug company can provide a royalty stream based on the sales of the drug instead of only on the software.
"A combination product is composed of at least two components reviewed by different centers at the FDA. Either a drug or biologics product used with a deice constitutes a combination product, or a drug and a biologics together ca be considered a combination product."
The labeling is very important as the generic drug company will not provide the software. However, the generic drug's label will instruct the patient to use the software, which courts have found to be an act of inducing infringement. The key for both the drug and software companies is the label. The drug label creates the combination and provides the evidence for infringement.
The combination of AI/ML software with a drug allows both companies to seek greater compensation for their efforts than either could achieve alone. This provides incentive for developing new treatment methods.
Originally published by Life Science Leader.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.

