Everybody has a type.
The Relationship Between Consumption Factors and Food Type Distribution Factors
In our last StepLadder article, we discussed consumption factors:
Consumption factors are values that represent "the fraction of the daily diet expected to contact specific packaging materials. The CF represents the ratio of the weight of all food contacting a specific packaging material to the weight of all food packaged."1Through the use of consumption factors, one is able to assess the upper-bound potential exposure to a food-contact substance in the diet, based on the calculated, estimated, or measured migration of the substance from packaging to food. If migration is the pie, dietary concentration is the slice of the pie.
It follows, then, that if dietary concentration is the slice of the pie, food type distribution factors (fT) are another way to slice that slice of the pie some more. (One of only a few instances in life where someone might want less pie!)
What are Food Type Distribution Factors?
Food type distribution factors (fT) are additional factors used in situations where a food contact material or article is specifically designed to contact certain foods, but not others, often (but not exclusively) where the upper-bound potential dietary concentration of the food-contact substance (FCS) is evaluated by way of worst-case (100%) calculations.2 As previously discussed in a StepLadder article on FDA's Food Types and Conditions of Use, a list of the US Food and Drug Administration’s (FDA) food types appear in its Chemistry Guidance document, Appendix V,3and can be more generally described as aqueous, acidic, alcoholic, and fatty. (FDA also recognizes dry food as a separate food type, though different assumptions apply). Since not all types of packaging contact all types of food, the use of food type distribution factors allows for more refined (and therefore, more accurate) assessments of the upper-bound potential exposure of a food contact substance (FCS) in the diet.
Similarly, FDA states in its Chemistry Guidance document that:
"Before migration levels can be combined with [consumption factor] (CF) values to derive estimates of probable exposure, the nature of the food that will likely contact the food-contact article containing the FCS must be known. Migration into a fatty-food simulant, for example, will be of little use in estimating probable exposure if the FCS is used exclusively in or for articles in contact with aqueous food. To account for the variable nature of food contacting each food-contact article, FDA has calculated "food-type distribution factors" (fT) for each packaging material to reflect the fraction of all food contacting each material that is aqueous, acidic, alcoholic and fatty.4"
Where food type distribution factors are not used, one effectively assumes that a food-contact material or article will contact all types of food, and therefore, the following applies:
Migration <M> × Consumption Factor (CF) =
Dietary Concentration (DC)
In contrast, where a food-contact material or article is intended to contact only one (or multiple, but not all) types of food, the calculation becomes:5
Migration <M> × Consumption Factor (CF) × Food Type Distribution Factor (fT) =
Dietary Concentration (DC’)
FDA’s food type distribution factors are repeated below for ready-reference:
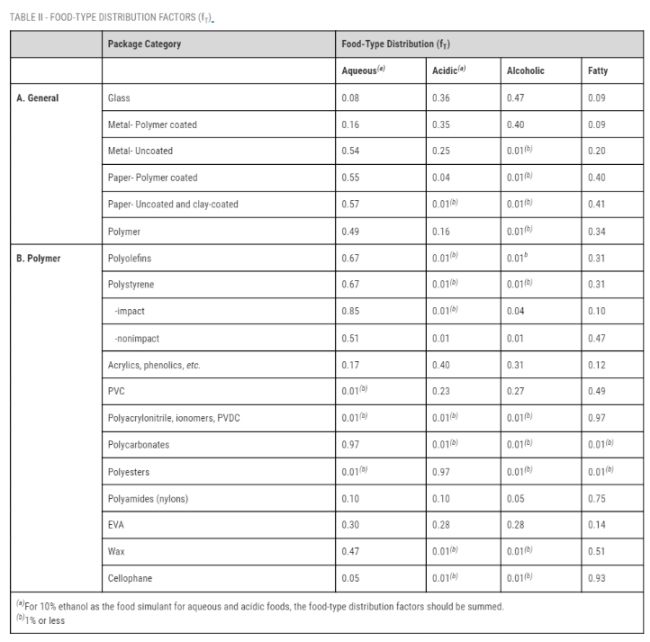
Courtesy U.S. Food and Drug Administration – website last visited September 4, 2023
Leveraging the same (fictional) example of how consumption factors may be used from our previous StepLadder article, an illustration of how food type distribution factors are used appears below, with the new step appearing in blue:
- An antioxidant is added to HDPE for use in contact only with fatty foods at a level of 0.025%.
- Based on additional factors (described in our "No Migration" StepLadder article), we determine through calculation that the upper-bound potential level of migration <M> of the antioxidant to food would not exceed 30 parts per billion (ppb).
- We then multiply the <M> value by the relevant CF for HDPE – 0.13 (13%) in this case – to calculate an upper-bound potential concentration of the antioxidant in the diet (the dietary concentration) in contact with all types of food of 30 ppb × 0.13 = 3.9 ppb.
- To account for the fatty food contact use (non-fatty food contact limitation), we then multiply the dietary concentration value by the food type distribution factor for polyolefins / fatty food (0.31) to obtain a dietary concentration from this specific, limited use (DC’) of 3.9 ppb × 0.31 = 1.21 ppb.
Depending on the toxicology profile of the substance, one could then consider the use of the migration value (<M> = 30 ppb) to establish a "No Migration" position for all foods, or alternatively, the dietary concentration (DC = 3.9 ppb) to reach a Generally Recognized as Safe (GRAS) determination for all foods, or the revised dietary concentration (DC = 1.21 ppb) to reach a GRAS determination for fatty foods only, as appropriate exemptions and exceptions to the need for premarket approval under the Act, respectively.6
Discussion
There are numerous advantages and disadvantages to using food type distribution factors when assessing the dietary exposure to a food contact material or article. The two most obvious are:
Advantage:
- Reduces the upper-bound (100%) worst-case dietary exposure value that needs to be supported from a safety and toxicology standpoint.
Disadvantage:
- Limits the applications for which the food contact material or article can be used; the marketing material may need to contain restrictive labeling, and/or these limitations may need to be communicated to downstream customers.
Importantly, the use of food type distribution factors (and accompanying food type limitations) can serve as an effective legal strategy for placing a food-contact material or article on the market for limited uses, based on readily-available and existing toxicology data where such uses represent a significant proportion of the actual or expected market. While this interim authorization or self-determination is in place, a company may then develop or obtain additional toxicology and safety studies to support expanded uses of the FCS moving forward.
Food type distribution factors also are particularly helpful in situations where an FCS is authorized under FDA’s food additive regulations for most – but not all – food types, and a company is interested in obtaining authorization for additional food type(s) where worst-case (100%) migration and dietary concentration calculations would necessarily over-estimate exposure.
A Note on Temperature Limitations
While not addressed in detail in this StepLadder article, companies should be aware that FDA does not publish analogous 'temperature distribution factors' – but that principles of diffusion may be used in certain instances to reduce the upper-bound (100%) potential calculated level of migration and corresponding dietary exposure for certain polymers. When actual migration studies are used in lieu of calculations, the migration protocol would simply utilize the most severe temperature conditions of expected contact, thereby potentially reducing overall migration of the FCS to the food-simulating solvent.
As with many principles of the regulation of food packaging, the use or omission of food type distribution factors should be made on a case-by-case basis, taking into consideration the various legal and marketing advantages and disadvantages that come with the application of such a limitation.
Footnotes
1. FDA Guidance for Industry: Preparation of Premarket Submissions for Food Contact Substances (Chemistry Recommendations), December 2007, accessible at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-preparation-premarket-submissions-food-contact-substances-chemistry
2.Food type distribution factors also are relevant when migration and corresponding dietary exposure is evaluated using actual migration studies, where individual migration values for each food simulating solvent are used proportionally (relative to the fT) to calculate a more refined, composite dietary concentration value.
3. Id.
4. Id.
5. Food type distribution factors can be summed across more than one food type, and can be used pro rata to evaluate exposure to one type of food versus another.
6. One could similarly use food type distribution factors in combination with consumption factors as a method of demonstrating that the presence of a residual impurity or byproduct is safe, and does not impact the suitable purity of the finished food-contact material or article.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.

