For a brief moment in time last April, the U.S. Food and Drug Administration's ("FDA") approval of the commonly-used abortion medication, Mifepristone, was curtailed. Just days after a Texas federal judge's ruling suspended the FDA's approval of the drug, the U.S. Department of Justice (the "DOJ") asked the Fifth Circuit to grant an emergency or administrative stay of that decision. On review, the Fifth Circuit held that Mifepristone could only be prescribed in the first seven weeks of pregnancy, under a physician's supervision, and the drug cannot be sent by mail, temporarily suspending more recent modifications to the FDA's approval.
The Fifth Circuit's decision was issued in the backdrop of another decision out of the Eastern District of Washington enjoining the FDA from altering the status quo as it relates to the availability of Mifepristone in seventeen states and Washington, D.C. Due to the "regulatory chaos" that ensued since the issuance of the Fifth Circuit order, Danco Laboratories, LLC ("Danco"), distributor of the drug, Mifepristone, sought emergency relief from the U.S. Supreme Court to either stay the Texas District Court's preliminary injunction in full pending appeal or grant certiorari in the case. The DOJ followed suit immediately thereafter, filing its own emergency request with the U.S. Supreme Court to restore access to the drug nationwide. Hours before the restrictions were set to go into effect, Supreme Court Justice Samuel Alito placed a five day administrative stay on the Fifth Circuit's order, and shortly thereafter, the Supreme Court granted the stay in full, preserving nationwide access to Mifepristone pending appeal.
Currently, the fate of Mifepristone lies with the Fifth Circuit as it considers whether to curtail the FDA's approval of the drug. It is anticipated that, regardless of how the Fifth Circuit rules, the decision will be appealed to the Supreme Court. Until such time, Mifepristone remains on the market without court-imposed restrictions. This article provides an in-depth analysis of the implications of the Texas case, including the availability and use of the Mifepristone drug going forward, and the potential impact to the FDA's broader authority, if the Texas plaintiffs ultimately prevail.
The Lifecycle of Mifepristone
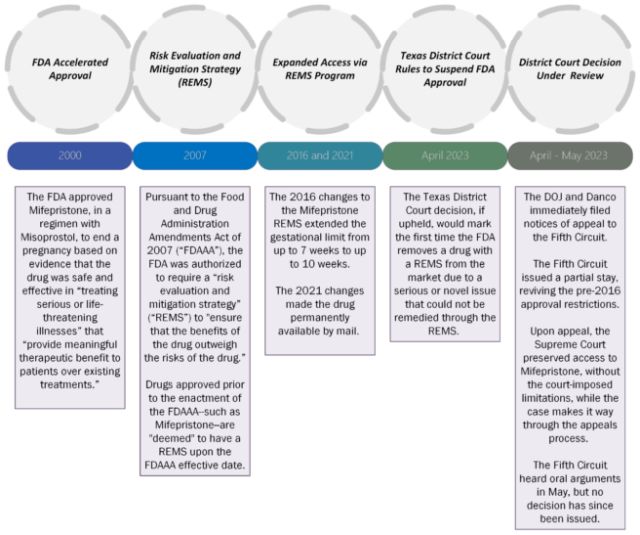
How did we get here? A Timeline
In the landmark decision, Dobbs v. Jackson Women's Health Organization, the Supreme Court overruled Roe v. Wade and Planned Parenthood v. Casey, holding that there is no constitutional right to abortion, and granting individual states the authority to regulate abortion as they deem appropriate.
In addition to a flurry of other lawsuits, legislative activity, and agency guidance, two federal cases playing out in the states of Washington and Texas are receiving national attention for the potential impact to the availability of the commonly used abortion medication, Mifepristone. These cases have been proceeding on roughly parallel tracks, with disparate outcomes:
| Washington Lawsuit [1] | Texas Lawsuit [2] |
| February 2023: A dozen democratic attorneys general file suit against the FDA in the Eastern District of Washington, challenging the FDA's current restrictions on Mifepristone, including distribution and certification requirements, as burdensome and unnecessary. The lawsuit seeks declaratory relief that Mifepristone is safe and effective, and that the FDA's approval of the drug is lawful. In its motion for preliminary injunction to enjoin the FDA from removing Mifepristone from the market, the plaintiffs note that "FDA's restrictions also single Mifepristone out for paper-trail requirements that create Orwellian dangers for patients and providers, potentially subjecting them to harassment, lawsuits, or even criminal prosecution." Mot. at 2. | November 2022: Alliance for Hippocratic Medicine, American Association of ProLife Obstetricians and Gynecologists, American College of Pediatricians, Christian Medical & Dental Associations, and individual providers (collectively, the "Texas Plaintiffs") file suit in the Northern District of Texas, challenging the FDA's decades old approval of Mifepristone under the Administrative Procedure Act (5 U.S.C. § 706). In their motion for preliminary injunction to withdraw or suspend the FDA approvals of chemical abortion drugs, the Texas Plaintiffs claim that the FDA is "running roughshod over the laws and regulations that govern the agency and, more importantly, protect the public from harmful drugs." Mot. at 2. |
| April 7, 2023: Judge Thomas Rice rules that the FDA is prohibited from "altering the status quo and rights as it relates to the availability of Mifepristone" in seventeen states and the District of Columbia. | April 7, 2023: Judge Matthew Kacsmaryk rules in favor of Plaintiffs, halting FDA's approval of Mifepristone, but stays the ruling for seven days in order to allow the Biden Administration time to seek an emergency appeal. |
| April 10, 2023: DOJ seeks clarification from the court regarding the FDA's obligations in light of the Texas ruling. | April 12, 2023: Fifth Circuit grants DOJ's request for a stay in part, allowing partial access to the abortion drug pending the appeal. The Fifth Circuit noted that while "the statute of limitations bars plaintiffs' challenges to the Food and Drug Administration's approval of Mifepristone in 2000," challenges to FDA's 2016 REMS changes to expand access and limit restrictions are in fact timely. Fifth Circuit Order, at 2. |
| April 13, 2023: In response to the request for clarification, Judge Rice issued an order affirming that for the 17 states and D.C., access to Mifepristone should remain unchanged, Texas ruling notwithstanding. | April 14, 2023:
Distributor of Mifepristone, Danco, Laboratories, LLC
("Danco") filed an application to the U.S. Supreme Court for
emergency relief, following the Fifth Circuit's order to
reinstate restrictions on Mifepristone during the appeal. Danco
requested either a full stay of the Texas District Court ruling
pending appeal or for the court to grant certiorari in the
case. April 14, 2023: Immediately after Danco filed its application, DOJ also sought emergency relief from the U.S. Supreme Court with the same basis as Danco. April 14, 2023: Supreme Court Justice Samuel Alito issued a five-day hold on the restrictions imposed under the Fifth Circuit order, giving the court until 11:59 pm ET on April 19th to fully consider the issue. |
| April 21, 2023: Following a two-day extension of the hold, the U.S. Supreme Court preserved nationwide access to Mifepristone by halting the Texas District Court's decision pending appeal. | |
| May 17, 2023: The Fifth Circuit heard oral arguments from the FDA, Danco, and the Plaintiffs. |
Had the U.S. Supreme Court affirmed the Fifth Circuit ruling, Mifepristone would have continued to be available for use while the Texas case was on appeal, but only for the first seven weeks of pregnancy and under physician supervision (as required under the pre-2016 REMS). In addition, Mifepristone would no longer be available via mail order while the Texas decision was being appealed. Moreover, the FDA, providers, manufacturers, distributors, and pharmacies with national operations would have been placed in the impossible position of having to comply with two directly conflicting orders. The Supreme Court's stay of the Fifth Circuit ruling has helped to avoid that issue for now, but the industry and patients who seek access to Mifepristone will be watching with bated breath for final resolution.
A Deep Dive into the Texas Case and its Implications if the Plaintiffs Prevail on their Claims
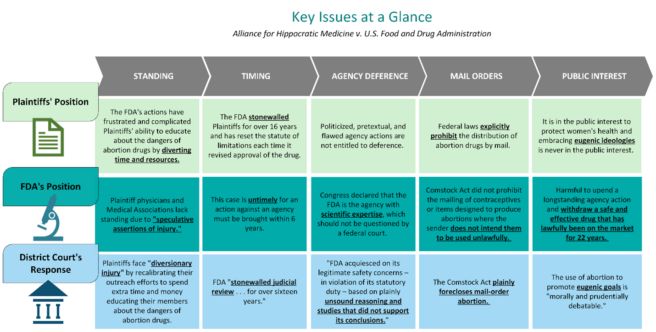
Implications of the Texas Ruling
In the aftermath of Dobbs, the potential withdrawal of Mifepristone not only impacts (and further restricts) access to abortion medication, but also creates uncertainty for providers and the FDA regulatory framework.
Accessibility: Medication abortions currently account for more than 50% of all abortions in the United States. If Mifepristone is no longer on the market or access is more limited, in states where abortion is legal, women may look to other forms of abortion, including surgical abortion, as an alternative, potentially increasing demands on already overburdened healthcare systems. Women who are at risk for complications from anesthesia or sedation may be compelled to use Misoprostol off-label, which may accelerate the termination timeline, compared to a Mifepristone/Misoprostol regimen. Although this off-label use may cause an abortion to occur faster, it carries the potential for complications.
Provider Uncertainty: In states where abortion is legal, adoption of a Misoprostol-only protocol may pose challenges. Some states adopt the position that medication abortions take place at the time the provider prescribes the medication, while other states claim that the medication abortion occurs when the pregnancy is terminated. This creates legal uncertainty for patients traveling between states where abortion is banned and states where abortion is legal and may impact how a provider instructs a patient to complete the Misoprostol regimen.
FDA Regulatory Framework: The Texas District Court's decision to withdraw Mifepristone relied heavily on the conclusion that abortion medication does not fall under the scope of the Accelerated Approval framework. Even though the FDA conducted a comprehensive scientific analysis of Mifepristone, and the Government Accountability Office published a 2008 report finding that FDA operated within their authority under Subpart H to approve the drug, the District Court chose not to defer to the FDA's expertise. The Texas ruling, if upheld, may undermine the FDA's statutory authority to evaluate and determine the safety and efficacy of drugs and medical devices through its longstanding regulatory approval pathways. This unprecedented ruling to withdraw an FDA-approved drug may lead to future challenges to other previously approved drugs, and may also cause uncertainty regarding the FDA's approval of new treatments.
Administrative Law: The Texas decision could have a significant impact on agency deference more generally, far beyond the FDA and its authority to regulate drug safety and efficacy. A federal agency's discretion to reasonably rely on data and specialized knowledge to support its decisions is a cornerstone of the administrative process. While judicial oversight is also a fundamental aspect of administrative law, courts have generally been reluctant to substitute their own judgment for that of an agency acting within its area of expertise. Indeed, nearly four decades ago, the Supreme Court ruled in Chevron v. Natural Resources Defense Council, Inc. that courts should give deference to an agency's interpretation of an ambiguous statute so long as that interpretation is reasonable. On May 1, 2023, the Supreme Court agreed to hear a case next term that explicitly challenges Chevron deference. While the Fifth Circuit in the Mifepristone case does not directly reference Chevron, it does so indirectly-by its failure to mention "deference" at all. Instead, the Fifth Circuit order solely invoked a constraint on agency decision-making authority: the arbitrary and capricious standard under the Administrative Procedure Act, which instructs courts to set aside agency decisions found to be unsupported by sufficient data. The Fifth Circuit's silence regarding application of the Chevron doctrine, may signal an impending judicial retreat from agency deference as a predominant feature of administrative law. This expansion of judicial authority, by the judiciary and through the overruling of Chevron, threatens the very existence of agency deference, which would undermine congressional delegation of authority to agencies armed with specialized knowledge, and weaken political accountability.
Recent Fifth Circuit Proceedings
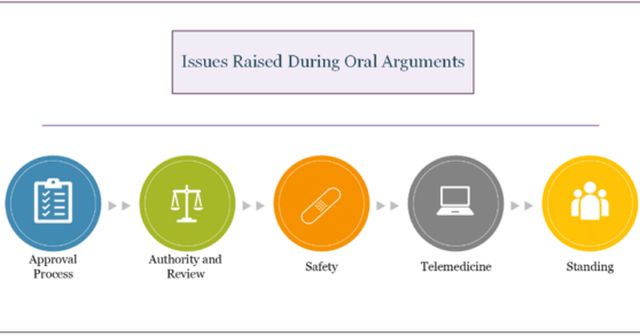
The Texas proceeding reached the Fifth Circuit for the second time on May 17, 2023, for oral arguments. The separate three-judge appellate panel was comprised of Judge James C. Ho, Judge Cory T. Wilson (both appointed by former President Donald Trump), and Judge Jennifer Walker Elrod (a George W. Bush appointee). During oral arguments, the panel scrutinized the FDA's approval of Mifepristone in 2000 and its decision to later relax certain prescribing requirements (e.g., allow distribution by mail). The panel questioned the safety and effectiveness of prescribing Mifepristone via telemedicine. Specifically, the panel sought clarification on overcoming state licensing barriers and how providers assessed their patients through a virtual or electronic platform, to which the FDA responded that the agency does not dictate the practice of medicine. The FDA clarified that it leaves the diagnostic and treatment decisions to the reasonable medical judgment of the provider based on the facts before them.
A large portion of the hearing also focused on the issue of standing and whether the Plaintiffs actually suffered a direct, concrete injury based on the FDA's actions. Additionally, the panel inquired into what the process entails for both the FDA and Danco to change the drug's labelling. This last inquiry signals a potential mixed ruling from the Fifth Circuit, as the panel may choose to keep the 2000 FDA approval of Mifepristone in place, while requiring the earlier restrictions on the drug to be re-implemented.
The Fifth Circuit has yet to issue a decision and there is no deadline to do so. Whatever the decision may be, the Fifth Circuit's ruling will be stayed if either party petitions the Supreme Court for a writ of certiorari and the Supreme Court grants certiorari, in which case, the case will move to the Supreme Court for review. If the Supreme Court denies certiorari, the stay on the Fifth Circuit's decision would be lifted.
What's Next?
Nationwide access to Mifepristone was temporarily preserved by the Supreme Court when it halted the Texas District Court's decision pending appeal. If the Fifth Circuit rules in the Texas Plaintiffs' favor, it is anticipated that the FDA will appeal to the Supreme Court. If the writ of certiorari is granted, the Supreme Court would once again take up the issue of abortion, as well as the extent of judicial deference to agency determinations.
When the Supreme Court decided Dobbs v. Jackson, it was in the abstract and on a theory that states should have the right to decide whether and how to restrict access to abortion. In the intervening year since that decision, a number of states have eliminated abortion access altogether, while others have increased access to abortion. In many cases, there have been clashes between conflicting state laws, resulting in some states reaching across their own borders to enforce their laws against citizens in other states. If the Supreme Court decides to take up the issue of Mifepristone in the near future, it will be interesting to see whether this new reality influences its ruling.
Sheppard Mullin's Women in Healthcare Leadership Collaborative ("WHLC") continues to closely monitor the docket and will provide updates as the case progresses.
This article was originally published on April 14, 2023, and updated on June 12, 2023.
Footnotes
1. State of Washington v. U.S. Food and Drug Administration, No. 1:23-cv-032026 (E.D. Wa. Apr. 7, 2023).
2. See Alliance for Hippocratic Medicine v. U.S. Food and Drug Administration, No. 2:22-CBV-223-Z (N.D. Tex. Apr. 7, 2023).
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.




