In a newsletter published at the end of 2020 on our website, we reported on two IP & Commercial Court (the IPC Court) decisions rendered in 2020, both holding that, in a patent term extension case, the "report date" rather than the "last date" (as argued by the IP Office) of a foreign clinical trial shall be deemed the "end date" of the trial.
These two patent term extension ("PTE") lawsuits now took a new turn. In Spring 2022, the Supreme Administrative Court (the SAC), namely the court of final instance for PTE disputes, held that the issue of calculating the duration of foreign clinical trials cannot be legally and appropriately addressed without advice from the Ministry of Health & Welfare (MHW). Taiwan IP Office v. Merck Sharp & Dohme B.V., 109 Shang 1045, SAC (May 2022). Taiwan IP Office v. F. Hoffmann-La Roche AG, 109 Shang 990, SAC (April 2022).
The two cases are now remanded back to the IPC Court.
What is the end date of a foreign clinical trial?
According to Article 4 of the Regulations for Ratifying Extension of Patent Term, the periods of time allowable in a PTE request include "the period of domestic and foreign clinical trials conducted for obtaining a pharmaceutical approval from the MHW" and "the examination period for domestic regulatory approval." Where a PTE request is granted, the patent term can be extended up to five years, but the extension is available only once (Article 53 of Taiwan's Patent Act).
The question is: what is the "end date" of a clinical trial, especially a foreign clinical trial, in a PTE case?
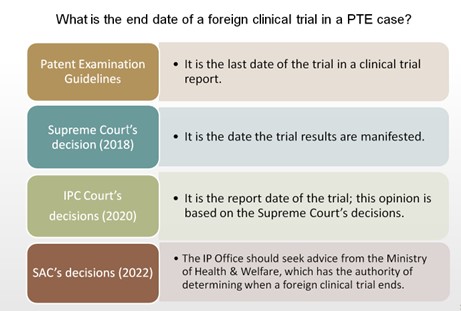
The IP Office's position: the last date of the trial
This question has been well considered by the IP Office, and its answer is put forth in the Patent Examination Guidelines, which stipulate that the last date of the trial referred to in a clinical trial report shall serve as the "end date." The text of the Examination Guidelines is self-explanatory and as follows:
When filing a patent term extension application based on a foreign clinical trial, the applicant shall state the key points of the clinical trial plan, . and record in a clinical trial report drafted in accordance with the ICH specifications the start and end dates of the trial, namely, the duration within which the trial is performed.
Supreme Court's notion in 2018: the date the trial results are manifested
However, not bound by the Examination Guidelines, courts dissent from the IP Office on this question. Firstly, in a civil judgment rendered in 2018 on an infringement dispute, the Supreme Court indicated that:
Conclusions of a clinical trial for a pharmaceutical product are not instantly obtainable after the pharmaceutical product is administered. Rather, the implications and conclusions of a clinical trial can only be manifested and reached after the drug administration conditions and the clinical responses are statistically analyzed and interpreted with professional knowledge, and only afterwards can a test result be submitted to the competent authority for examination on whether to issue a marketing approval. Therefore, to meet the legislative purpose, the period of a clinical trial should be interpreted as starting from the date the clinical trial is commenced and ending on the date the trial results are manifested. Pfizer Ireland Pharmaceuticials v. Nang Kuang Pharmaceutical Co., Ltd., 106 Tai-Shang 1904, Taiwan's Supreme Court (2018).
The IPC Court's decisions in 2020: the report date
Two years later, in June 2020, this view was largely adopted by the IPC Court in the two decisions mentioned in the first paragraph of this Newsletter. Merck Sharp & Dohme and F. Hoffmann-La Roche each convinced the court that the end date of a foreign clinical trial in a PTE case should be the "report date" rather than the last date of the trial (see our Newsletter for more details.)
The IP Office did not amend the Examination Guidelines or any sister bylaws to accommodate this court view. Instead, they appealed the IPC Court's decisions to the SAC, which is rare.
The SAC's opinions in 2022
Rarer still is the SAC's approach to address this issue. On the one hand, the SAC touched on the subtle difference between the "report date" and the date the trial results are manifested. It constitutes a legal error, the SAC said, to count in the period of preparing a trial report when calculating the duration of a clinical trial, where, as here, there is not a legal source that supports this notion. The IPC Court's decision was thus revoked and remanded.
However, the SAC did not reject altogether the possibility of equating the end date of a foreign clinical trial with its report date. In both of these decisions, the SAC reminded the parties, i.e. the IP Office and the patentees, that whether a period of an activity in the course of a clinical trial is required for obtaining a marketing approval is a question to be answered by MHW; namely, it is MHW that holds the authority of determining where and when a foreign clinical trial ends. So, when examining a PTE request, the IP Office should refer the case to MHW for their advice, the SAC held. This seems to imply that, if MHW confirms that an activity (e.g. preparing a clinical trial report) and its length of time are needed for obtaining a marketing approval, then they would still have a good chance to be considered in a PTE case.
On the other hand, for the same reason, the SAC did not accept the IP Office's argument that a foreign clinical trial's end date is the last date of its trial. While this makes the SAC's holding look similar to the Supreme Court's decision of 2018, the SAC's rationale was a purely procedural one: the current Patent Examination Guidelines on PTE applications were drafted and applied without first seeking MHW's advice.
Leaving aside the uncertainties and complexities of interactions between multiple government agencies, what will SAC say if the MHW endorses the Patent Examination Guidelines and agrees that, at least in PTE cases, a foreign clinical trial should end on the last date of the trial referred to in the clinical trial report? We might never know the answer, because, according to a proposed overhaul of Taiwan's Patent Act, SAC is going to transfer its jurisdiction over administrative IP disputes to the Supreme Court entirely (see our report about this proposed amendment.) If the two cases reported in this article are appealed once again to the court of final instance, say, two years from now, it is possible that the court of final instance at that time will become the Supreme Court. It is possible that the current deadlock about PTE requests based on foreign clinical trials will remain unbroken until that time.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.
