The public expenditure on medicines sold in pharmacies increased significantly in 2022 when compared to the previous year. At the same time, there were shortages of some medicines in pharmacies and, in many cases, this led to consumption being diverted to more expensive alternative medicines.
Administrative Regulation 35/20231 has been published to deal with this situation, and it:
i) Updates the reference countries to be taken into account in 2023 when defining the prices of new medicines and in the annual revision of the prices of medicines purchased by institutions and departments of the National Health Service and of medicines dispensed in the outpatient market; and
ii) Establishes exceptional criteria to be followed in the procedures for the annual review of the price of medicines, thus allowing an increase in the retail price of medicines with a price of EUR 15.00 or less.
Reference countries for new prices and for the annual price revision
- In 2023, the reference countries of the previous year are maintained: Spain, France, Italy and Slovenia.
Exceptional criteria to be observed in the Annual Review of Medicine Prices (APR) 2023
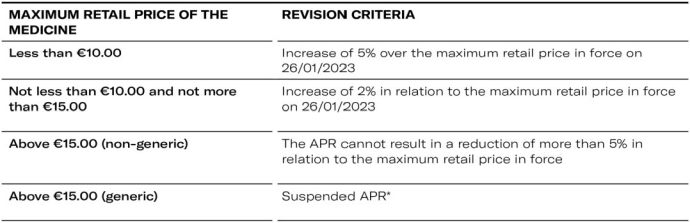
- Hospital Market - Suspended APR*
* Excluded from the suspension are generic medicines (outpatient and hospital markets) whose maximum price is higher than the maximum price of the reference medicine resulting from the 2023 APR or, if applicable, the 2023 price increase, in which case the maximum price of the generic medicine may not exceed the latter values.
Deadlines for marketing authorisation holders or their
legal representatives to submit lists of prices to be applied in
2023:
- Annual review of the maximum retail price of non-generic medicines: By 15 February 2023, with prices taking effect on the following 1 March
- Annual review of maximum retail price for generic medicines: By 15 March 2023, with prices coming into effect on the following 1 April
List of essential medicines
- By 26 April 2023, INFARMED is expected to draw up a list of essential medicines whose criticality may justify the application of specific measures, including an increase in the maximum price.
Extraordinary price review
- In addition to the above review, it is expected that there may be an extraordinary review of the prices of medicines on the grounds they are excessively costly to the NHS.
If this occurs, this extraordinary review will be decided by the member of the Government with responsibility for health, who will also determine the criteria for determining what is considered 'excessive cost'.
Footnotes
1 Ministerial Order 35/2023 was published on 26 January 2023 and came into force the following day. Note that this Ministerial Order was subject to a declaration of rectification (Declaration of Rectification 6/2023 of 6 February).
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.



