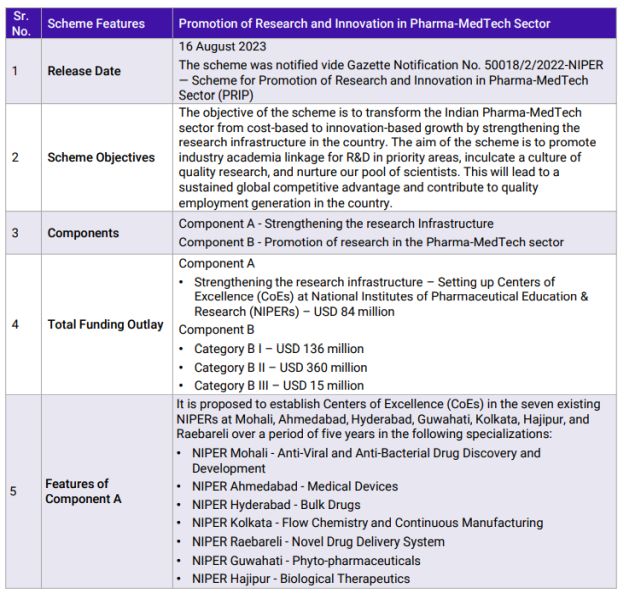
The Government of India (GoI) has focused on encouraging manufacturing in India and introducing innovative technologies. It has been instrumental in encouraging industries to 'Make in India' for domestic consumption and the world at large. The GoI has been regularly taking policy and regulatory interventions to help the industry manufacture and innovate in India. The AtmaNirbhar Bharat initiative, Production Linked Incentive Schemes, National Medical Device Policy, and New Drugs and Cosmetics Act (Draft) are examples of such initiatives. Taking forward this approach, the GoI last month announced the Promotion of Research and Innovation in Pharma-MedTech (PRIP) Scheme 2023. Under this scheme, the GoI has allocated USD 600 million over a five-year span for research and innovation activities in the field of Pharmaceuticals and Medical Devices.
Pharmaceuticals is a research-intensive industry and requires continued Research and Development (R&D) to not only remain competitive but also to grow the industry further. The Indian Pharmaceutical industry has accomplished significant milestones over the past four decades, the biggest of them being dubbed as the 'Pharmacy of the World'.
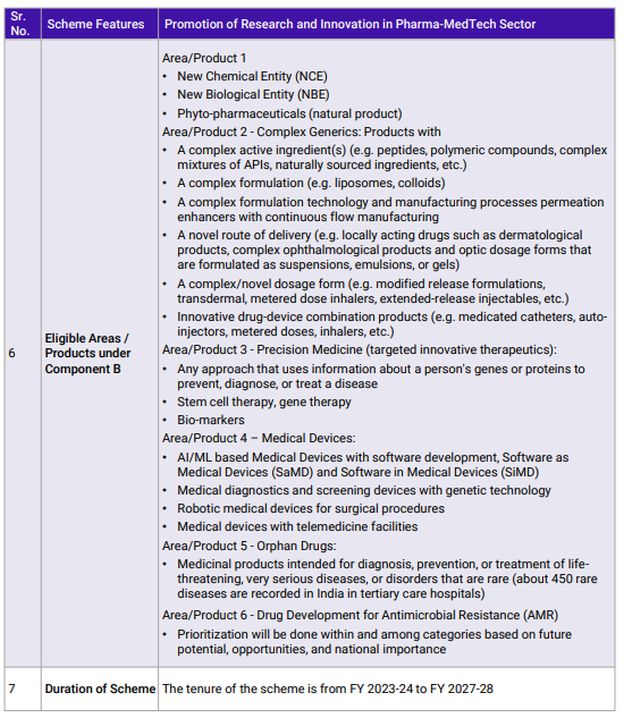
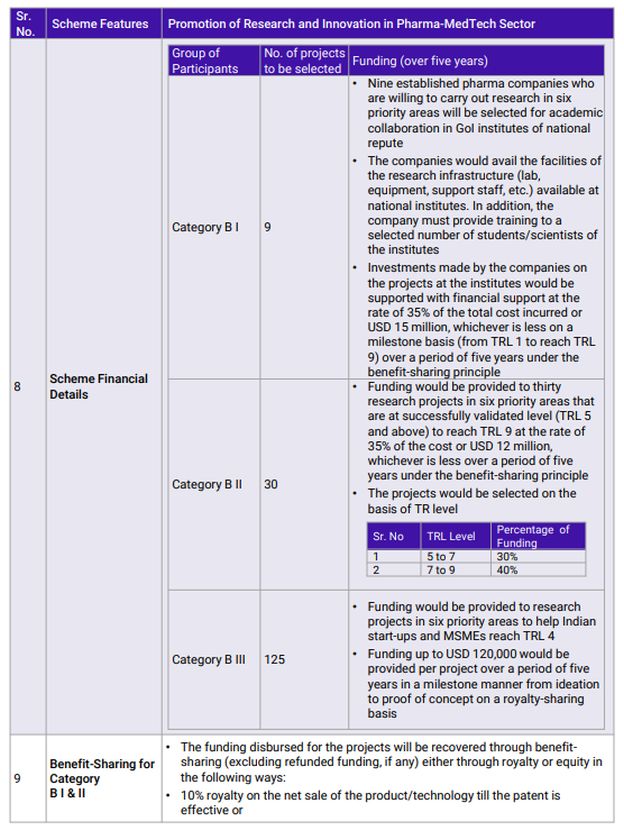
Over this period, Indian pharmaceutical companies have focused on generic drugs and held leadership for over two decades. The generic drug industry, while being complex, does not require R&D in comparison to patented drugs or creating New Chemical Entities (NCEs). Hence, the R&D spend has hovered around 3-5% of sales at industry levels which rises up to 7% for the Top 10 companies. There is an urgent need to increase this spending significantly to not only remain competitive but also to take the next step in the value chain. Recognizing this need, the GoI has identified five priority areas that hold the potential for the future and will help the industry leapfrog into the future.
The PRIP scheme also covers the Medical Devices industry. Along with the Pharmaceuticals industry, Medical Devices are an integral part of the Healthcare sector. The industry currently has a market size of around USD 11 billion and is expected to reach USD 50 billion by 2047. The industry has witnessed sustained growth if we exclude the COVID years. It is expected to continue to grow in the range of 15-18% CAGR for the next decade. It is an import-dependent industry with more than 70% of devices being imported even now. To help reduce this number, the GoI has taken several steps as they did for pharmaceuticals (devising New Medical Devices Rules, 2017 and the Medical Device Policy, 2023, and topping it up with Production Linked Incentives (PLIs) to encourage domestic manufacturing. The missing piece in this reform was R&D. With the PRIP scheme, the government is addressing that need while also providing the industry with an opportunity to lead the world in affordable but innovative technologies in the field of Medical Devices.
We have compiled a list of key features of the scheme below:
Conclusion
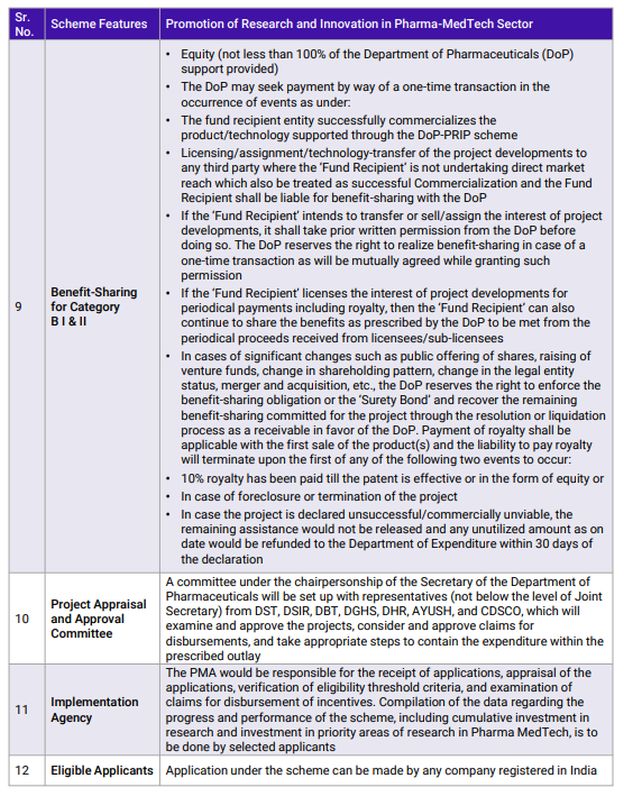
Any nation's development is built on foundations of innovation. A nation that does not encourage research and development will not climb the development ladder quickly. While India has been conducting R&D at its own pace considering the restraints of resources and knowledge, it needed a catalyst to accelerate development. The PRIP scheme will act as catalyst that will expedite R&D in the country and more importantly, build a culture of sustained research and innovation. This also marks a paradigm shift in the Healthcare sector from a foundation of cost-centric competitiveness to one that will progress on innovation-driven growth. The policy takes a first principles approach of understanding the basics of Pharmaceuticals and Medical Devices products in an academic environment and then building on that knowledge by collaborating with the industry, leading to innovative commercial products. Establishing specific CoEs at NIPERs with in-house research graduates will also assist the Pharmaceutical and Medical Devices industry with skilled manpower for continuing R&D efforts. Component B of the scheme covers everything from complex generics to NCEs, providing ample opportunities to organizations from across the spectrum to participate and innovate in India.
The PRIP scheme in conjunction with the Bulk Drug and Medical Device Parks alongside PLI schemes will augment the entire value chain of the domestic manufacturing industry. The GoI has provided the Healthcare sector with a strong base to not only maintain its leadership position in Pharmaceuticals but also emerge as a strong player in Medical Devices industry. It is crucial to mention that such initiatives open India to the world in the form of skilled labor, increased capital inflows, and shifting manufacturing bases as over the next few years, India will have a complete ecosystem for healthcare.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.
