Abstract
In recent years, the amount of patent infringement litigations among pharmaceutical companies have been increasing year by year, which creates barriers for the drug marketing. To change this situation, it is necessary to evaluate the patent infringement risk by conducting freedom to operate (FTO) patent search and analysis, and generate corresponding response strategy based on the evaluation results to reduce related IP risk.
Text
The objective of FTO patent search and analysis in the pharmaceutical field is to find out the obstacle patents of risk being infringed as comprehensively and accurately as possible, so as to effectively prevent legal risks. FTO patent search and analysis is a multi-link process, usually including patent search, patent screening, patent infringement analysis, conclusions and recommendations, etc., which will be described in detail below.
I、When to conduct the FTO investigation in the pharmaceutical field
1. Before the approval of a research project or during the research and development process
FTO investigation at this stage can help pharmaceutical companies understand the patent landscape of drugs and provide guidance and advice on the establishment, research and development of a drug project. However, because the drug design plan has not yet been formulated at this stage, the FTO investigation would not be much targeted.
2. After the completion of drug development and before marketing
FTO investigation at this stage can help pharmaceutical companies understand when and where to put the drugs on the market. Since the drug design plan has been basically completed at this stage, FTO investigation is often very targeted.
3. Financing, mergers and acquisitions (M&A), licensing and assignment of pharmaceutical companies
In the financing or M&A process of pharmaceutical companies, the FTO investigation is mainly used to help investors or acquirers to assess the value of drugs and transaction risk; while during the licensing or assigning process of the drug project, FTO investigation aims to help licensees to evaluate the drug value and the associated transaction risk.
II、FTO Patent Search
It is required to find out all the related patents in the FTO investigation. Once the patent search is not sufficient and comprehensive, it will increase the infringement risk to some extent. Therefore, it is crucial to find out all the patents of risk by making proper searching strategy to ensure the comprehensiveness.
For the chemical drug, patents that would affect the freedom-to-operate of drugs may include several types, such as compounds, salt forms, crystal forms, preparation methods, uses, combinations, and dosage forms. The claims of patents or patent applications in the chemical field are sometimes written using the broader concept of the compound, which should be reasonably considered when conducting the patent search. For example, assume that the target drug is an EGFR inhibitor, and then EGFR can be used as a key word in the FTO patent search. In addition, since there's certain limitation when searching patents in the chemical field only using keywords, it is necessary to do the search in combination with the drug structural formula.
For the bio-pharmaceutical drug, patents that would affect the freedom-to-operate of drugs may also include several types, such as antigens, targets, epitopes, nucleic acids, proteins, expression vectors, expression cells, preparation methods, uses, combinations, and dosage forms. Claims in patents or patent applications in the bio-pharmaceutical field are sometimes written using broader concepts such as family, ligand and receptor, which should therefore be reasonably considered in many aspects when conducting the patent search. In addition to the use of keywords, FTO patent search should also combine the bio-sequence of the drug in the bio-pharmaceutical field.
1. Search basic information of drug
Before using a specialized search tool for patent search, it is common to use the drug information retrieval tool to search for basic information, including the Chinese and English names of the drug, Chinese and English extension names, Chinese and English trade names, chemical structural formulas, biological sequences, molecular formulas, CAS number, targets and indications. Common information retrieval tools for commonly used drugs include the company's official website, Google, FDA, NMPA, Drugfuture, DrugBank, PubChem, and Adis Insight, etc.
2. Select patent search database
The patent database is determined based on the target country of the search and the drug type. Generally speaking, if searching is based on English keywords and applicants, it is recommended to use foreign commercial patent databases such as Thomson Innovation, Total Patent or PatBase; if completely based on Chinese keywords or applicants, it is recommended to use the database provided by SIPO or the CNIPR database introduced by China Intellectual Property Publishing House; if it is a patent search for the compound structure, Scifinder search tool is generally used; if searching the biological sequence, SNT, Genome Quest or BLAST of NCBI are generally used.
3. Patent search element list
The patent search element list generally includes Chinese keywords, English keywords, patent classification numbers, chemical structures, biological sequences, applicants and inventors.
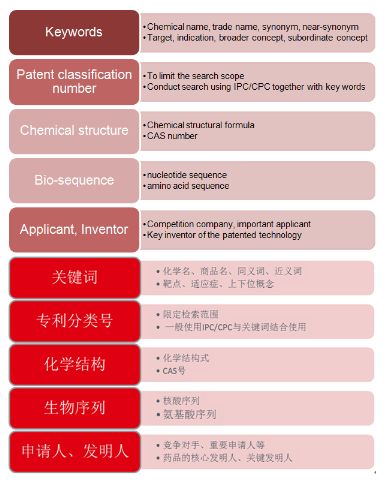
It should be noted that since the description of the patent infringement risk is not necessarily a well-known keyword, the expansion of the keyword is not only obtained in the general information of the drug, but also supplemented in the process of reading the patent document. For the patent classification number, it is generally determined through comparison based on statistical methods. Meanwhile, attention should also be paid to the classification number of the highly relevant patent in the process of reading the patent documents, and whether there's any missing among the previously identified classification numbers, if yes, prompt supplement is necessary.
4. Design searching formula
When constructing a search formula, a variety of patent search methods are often used, so as to better ensure the comprehensiveness of results. Firstly, it is necessary to use the drug-related keywords to search. When a large number of unrelated interference patents appear in the patent search results, in addition to adjusting the keywords, the classification number is needed to be used to further narrow the results; secondly, the specific database should be used for chemical structure or bio-sequence search; finally, the applicant and the inventor are used to perform a supplementary patent search.
III、 FTO Patent Screening
Patent screening can be performed in two stages, preliminary screening and precise screening. In the preliminary screening, the reverse exclusion method is generally adopted, i.e., the patents obviously irrelevant are excluded, and the patents with doubts are all retained, and the protection scope and different versions of the patent may not be considered at this stage. In the precise screening, the technical solutions protected by the independent claims in the preliminary screening list would be compared with the technical facts, and then select highly relevant patents by judging the correlation of the technologies as a whole.
It is worth noting that for FTO patent search in China, it can be screened and excluded through the application date and legal status, i.e., patents or patent applications that have been applied for more than 20 years from the application date, or invalid patents can be excluded. For FTO patent search in foreign countries or regions, please keep in mind do not judge whether it is expired based on the application date plus 20 years. Instead, before making a judgment, you should know the calculation method of patent expiration date in each country or region. For example, for US drug patent applications, there are two systems for drug extension protection period (PTA+PTE), and the specific patent expiration date can be obtained from Orange Book or USPTO.
IV、Patent Infringement Analysis
The patent infringement analysis mainly includes the break-up of the technical features of the claims, determination of the protection scope of the claims, break-up of the technical features of target drugs, and comparison analysis of the patent infringement risk.
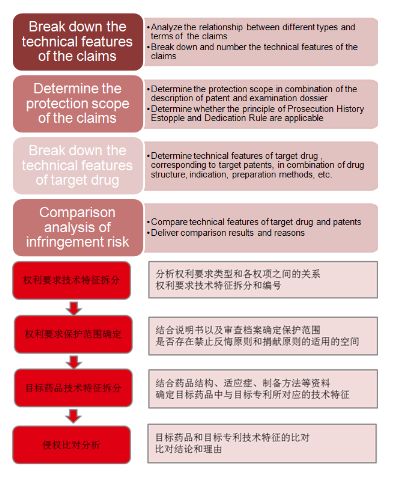
1. Break down the technical features
Analyze the relationship between the types of independent claims and terms of the claims, split the technical features in the independent claims, and number them to facilitate subsequent comparison analysis of patent infringement risk.
2. Determine the protection scope of claims
Firstly, to understand and grasp the specific meanings of the specific technical features in the claims based on the background technology and the description of the target patent document; secondly, retrieve the examination files of the target patents through the official website of the national patent offices, including Notification of an Office Action, Applicant's Response for the Office Action, Re-examination Dossier, Request for Invalidation, Examination Decision on Request for Invalidation, etc., study the response documents of the target patent in the process of confirming rights or safeguarding rights, determine the interpretation of the technical features by the applicant or patentee and the impact on the protection scope, and determine whether the Prosecution History Estoppel is applicable; finally, determine whether the Dedication Rule is applicable by studying the various embodiments in the patent description and analyzing the relationship between all the embodiments and the claims.
3. Break down technical features of target drug
Determine the technical features of the target drug corresponding to the target patent by combining the information of drug structure, indications, preparation methods, etc., and number the technical features to facilitate subsequent patent infringement comparison analysis.
4. Patent infringement comparison analysis
Compare the technical features of the target patent with that of the target drug one by one in the form of a list and deliver the comparison conclusions and brief reasons of each pair of technical features, then analyze and judge the patent infringement risk according to the rule of Literal Infringement and Doctrine of Equivalents, so as to determine whether the target drug falls within the protection scope of the target patent after comprehensively analyzing the judgment results.
Since compound patents are unique compared to other patents, some basic principles for infringement determination of compound patent will be specifically described below.
1) If the target patent protects drug in the form of a chemical name, the chemical name may correspond to a class of compounds or a kind of specific compound. In the case of a class of compounds, as long as the target drug belongs to one of the compounds, it can be considered as falling within the protection scope of the target patent; in the case of a kind of specific compound, it will be recognized as falling into the protection scope of the target patent only if the target drug is the compound itself.
2) If the target patent protects the compound in the form of the structural formula, its various functional groups and molecular stereo configuration should be focused on. If any functional group changes at the atomic level, it can be considered as no infringement risk, because any change in the functional group will lead to the production of another substance. In addition, as changes in the stereo configuration of the molecule would also result in different protection scope, in the case that the target patent claim specifically protects a specific molecular configuration, other molecular configuration except the protected configuration does not have the infringement risk.
3) If the target patent protects the molecular formula of a compound, various isomers of the compound own the infringement risk. If the molecular formula of target drug changes at the atomic level compared with that of the target patent, there is no patent infringement risk.
V、Conclusions and Advices
According to the results of patent infringement analysis, if there are high-risk patents, countermeasures should be made to avoid pharmaceutical companies falling into patent infringement litigation or transaction disputes. The general response strategy includes the following methods.
1. Bypass the high-risk patents
It could be considered to bypass the protection scope of infringing patents by improving drug development solutions. Generally speaking, patents protecting compounds or indication would generally have no chance to be bypassed, but patents protecting crystal, preparation patents, and preparation method can be effectively bypassed. For the crystal type patent, it is suggested that the clients could try to develop new crystal form to bypass the infringing patents, provided that the new crystal form is bioequivalent to the original crystal form of the drug. For the patent protecting the drug formulation, it can effectively bypass the target patents by reducing or changing the type or proportion of the auxiliary materials. For the preparation method patent, it can effectively bypass the target patents by changing the synthetic route or reaction conditions.
2. Request for Invalidation of obstacle patent
For patents or patent applications that are difficult to be bypassed by changing drug design schemes, if their stability or patentability is poor, it is recommended to submit an invalidation request or a third party public opinion. In general, the patents for crystalline forms, patents for dosage forms, and patents for therapeutic methods are more likely to be invalidated.
3. Waiting for the expiration of obstacle patents
For patents with strong protection and no way to be bypassed, such as compound patents, it is generally recommended to wait directly for the expiration of the patent. It should be noted that the expiration date of compound patent is generally calculated according to the specific compound patent applied later, which should be pay more attention to.
4. Licensing or transfer
In the situation that the patent is difficult to be bypassed and the patentee may license or transfer, for example, the patentee is a university, or the main business is not related to the patent, it is recommended that the client could negotiate with the patentee on the patent license or transfer to ensure the freedom-to-operate of patent. .
Conclusion
It can be seen from the above analysis that whether the expression of FTO patent search elements, the selection of patent search databases, the formulation of search terms and the screening of patent search results, or patent infringement analysis, require the patience and meticulousness of patent analysts, only by which could better assess the potential risks of pharmaceutical companies in the process of drug research and development, marketing, licensing or financing and M&A.
Footnotes
1. 韩羽枫. 侵权判定原则在化合物专利案件中的适用[J]. 商法专栏: 知识产权诉讼与执行, 2014, (10): 62-63.
2. 路炜, 肖沪卫. 专利侵权检索与分析报告的规范研究[J].图书情报工作,7714 2008,52(2):73-73.
3. 龙克文. 企业专利信息利用和防侵权检索分析[J]. 中外企业家, 2013 (10): 12.
4. 王吉霞. 专利防侵权调查的检索方法探究[J]. 现代情报, 2014, 34(6): 95-98.
5. What is a freedom to operate (FTO) or infringement search?
6. IP and Business: Launching a New Product: freedom to operate. WIP0.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.

