On October 8, 2023, the Ministry of Science and Technology, in collaboration with nine other national departments, unveiled the Measures for Review of Scientific and Technological Ethics (for Trial Implementation) (the "Measures"). The Measures aims to lay down a solid ethical framework for scientific research and technological development, providing comprehensive and uniform ethical review standards across sectors.
This move was precipitated by concerns voiced by the General Office of the State Council in March 2022 during their efforts to address the intricate challenges of upholding ethical standards in the face of rapid technological progress. Their concerns spotlighted challenges such as oversight deficiencies and inconsistencies in governance across different science-tech sectors. Considering this, a range of national departments and regions, including the National Health Commission and provinces like Beijing and Guangdong, rolled out their own sectorial or regional science-tech ethical governance initiatives. The introduction of the Measures signifies the commitment on a national level to preserving ethical standards in an era marked by swift technological metamorphosis. This article will address pivotal FAQs regarding the Measures.
1. WHAT ARE THE TRIGGERING CONDITIONS OF SCIENCE-TECH ETHICS REVIEW?
Article 2 of the Measures outlines four categories of innovative activities that mandate a science-tech ethics review:
- Activities that involve humans as research participants. This encompasses experiments where humans are tested, investigated, observed, or serve in a similar capacity. It also includes activities using human biological samples and personal data.
- Activities that involve experimental animals.
- Activities that, while not directly involving humans or experimental animals, may present ethical risks to life and health, the ecological environment, public order, sustainable development, and so forth.
- Any other activities that law, administrative regulations, or other relevant state rules require a science-tech ethics review.
The reference to "laws, administrative regulations, or other relevant state rules" in the fourth category, at a minimum, relates to the following provisions:
- Article 7 of the Administrative Provisions on Recommendation Algorithms in Internet-based Information Services mandates that recommendation algorithm providers put in place both management systems and technical measures dedicated to science-tech ethics review.
- Article 7 of the Administrative Provisions on Deep Synthesis in Internet-based Information Services requires that deep synthesis service providers establish a robust science-tech ethics review management system.
- Article 8 of the Implementation Rules for the Regulations on the Management of Human Genetic Resources specifies that any collection, storage, usage, and offshore allocation of China's human genetic resources must first undergo and passa science-tech ethics review overseen by qualified science-tech ethics committees.
- Article 9 of the Administrative Measures for Personal Information Protection Compliance Audits (Draft for Comment) highlights that for automated decision-making systems processing personal information, an essential component of the compliance audit is to verify if the algorithmic model has successfully undergone a science-tech ethics review.
2. WHO INITIATES THE SCIENCE-TECH ETHICS REVIEW?
Entities, ranging from higher education academies and research institutes to medical facilities and corporations, bear the primary responsibility for conducting science-tech ethics reviews. Within these entities, the initiation of science-tech reviews typically falls under the purview of their designated science-tech ethics committees. To facilitate this, entities are obligated to equip such committees with the necessary personnel, workspace, and funding. Moreover, necessary measures must be in place to ensure that the science-tech ethics committee operates with full autonomy during its review processes.
In practice, the establishment of a science-tech ethics committee is indispensable for any entity tasked with such ethical review responsibilities. The Measures stipulates that enterprises involved in fields like life sciences, medicine, and AI have legal obligations to set up such ethics committees. Notably, for AI enterprises aiming to successfully register their algorithms, the presence of an active and functional science-tech ethics committee becomes a vital assessment criterion. Furthermore, even for entities without legal obligations to form their own ethics committees, the Measures still advocates for a collaboration approach: these entities are expected to formally delegate their ethics review responsibilities to an already-established and compliant science-tech ethics committee outside their organizations.
3. WHAT ARE THE MEASURES REQUIRED FOR THE SCIENCE-TECH ETHICS COMMITTEE?
The Measures has set forth explicit criteria regarding the set-up of the science-tech ethics committee:
- Number of Members: The committee should be composed of at least seven members, which includes one chairperson and multiple vice-chairpersons.
- Background and Diversity: Members should represent a diverse background, encompassing individuals not affiliated with the primary institution, representatives of different genders, and individuals familiar with the intricacies of ethnic autonomous prefectures, particularly if the committee's work is relevant to those regions.
- Qualifications: The committee should comprise professionals familiar with relevant scientific and technological disciplines, alongside experts in ethics and law. All these members are expected to uphold the highest standards of research integrity.
- Tenure: Members should serve for a term not exceeding five years, but reappointment for consecutive terms is permissible.
4. WHAT DOCUMENTS MUST BE REVIEWED BY THE SCIENCE-TECH ETHICS COMMITTEE?
Article 9 of the Measures specifies six sets of documents that must be furnished to the science-tech ethics committee for review:
- Activity Profile: This includes the initiative's name, its objectives, significance, reasons for its undertaking, and any prior ethical reviews for such initiative.
- Operational Blueprint: A strategic layout for executing the science-tech activity. This covers the project's blueprint, potential ethical risks, risk mitigation strategies, contingency procedures, and the proposed method for disseminating results.
- Organizational Credentials: Documentation capturing the credentials of all participating bodies, the research experience of involved individuals, details of their past training regarding science-tech ethics, funding origins, and any declarations addressing potential conflicts of interest.
- Consent & Origins: An informed consent form, along with descriptions about where biological samples, data sets, and experimental animals (if used) are sourced from.
- Commitment Letter: A formal declaration underscoring a commitment to uphold science-tech ethical standards and maintain research integrity.
- Supplementary Materials: Any other materials that the science-tech ethics committee deems pertinent for a thorough review.
The decision on whether to proceed with an application rests with the science-tech ethics committee and hinges on the comprehensiveness of the submitted materials. Should there be gaps or omissions, the committee shall outline all additional requirements during a single communication.
5. WHAT DO DIFFERENT TYPES OF SCIENCE-TECH ETHICS REVIEW PROCEDURES ENTAIL?
There are three distinct science-tech ethics review procedures: the Summary Procedure, the Standard Procedure, and the Special Procedure which incorporates an expert second review. Each procedure varies in its initiation criteria, review panel composition, decision-making mechanism, and frequency of follow-up reviews. A detailed comparison is provided in the table below:
|
Criteria/Procedure |
Summary Procedure |
Standard Procedure |
Special Procedure |
|
Initiation Criteria |
|
Applicable to all science-tech activities not subject to the Summary Procedure. |
This procedure is geared toward new science-tech initiatives that potentially carry substantial ethical challenges. The specificities are outlined in the List of Scientific and Technological Activities Requiring Ethical Examination and Review which describes what activities mandate expert second review |
|
Review Panel Makeup |
The committee's chair selects at least two members to undertake the review. |
The review is chaired by the main chairperson or a designated vice-chair. The review meeting should have a minimum attendance of five members, representing a broad range of backgrounds and areas of expertise. |
For the preliminary review, the structure mirrors the standard procedure. For the expert stage, there's a shift to a panel made up of over five specialists, without involving the standard committee members. |
|
Decision Framework |
A unanimous agreement is required. If unanimous agreement is not achieved, the review defaults to the standard procedure. |
More than two-thirds of the participating members must grant their approval. |
For the preliminary stage, more than two-thirds of the attendees need to concur. For the expert second review, the agreement must come from more than two-thirds of the convened experts. |
|
Frequency of Follow-up Review |
The committee may adjust the follow-up review intervals as deemed appropriate. |
The follow-up review typically takes place within a 12-month timeframe. |
The follow-up review is usually set for every 6 months. |
*Minimum risk refers to everyday risks that one might encounter
during their daily life or risks comparable to health
checkups.
The List of Scientific and Technological Activities Requiring Ethical Examination and Review (the "List"), as mentioned in the above table, serves as a pivotal reference for the Special Procedure. The List will be maintained and updated from time to time by the Ministry of Science and Technology. Activities enumerated in The List must first undergo a preliminary review by the science-tech ethics committee. Once the preliminary review is complete, such review then must be reported to either local supervisory bodies or relevant industry departments for a thorough expert second review.
The criteria determining whether a science-tech activity will be included in the List revolve around three key risk considerations.
- Inherent Ethical Risks of the Technology should be considered, which encompasses the comprehensiveness of scientific knowledge and the extent of available safety information, as well as the technology's maturity, operability, safety, efficacy, and controllability.
- Factors such as potential ethical risks of the activity, the likelihood of ethical complications, the nature and severity of potential risks, and the extent of their impact are evaluated under this category.
- Justifiability and necessity of the activity, its intended audience, or target application scenarios, among other factors, should also be considered.
Specifically, according to the Measures, the following activities—when appearing on the List—necessitate an expert second review after the initial ethics review:
- The research focused on synthesizing new species with potentially far-reaching impacts on human life, health, our ecological environment, or foundational societal values.
- Exploration into the insertion of human stem cells into animal embryos or fetuses, subsequently developing within an animal uterus.
- Fundamental studies into altering the genetic makeup or inheritance processes of human reproductive cells, fertilized ova, or pre-implantation embryonic cells.
- Clinical trials of invasive brain-machine interfaces targeting neurological or psychiatric ailments.
- R&D initiatives centered around human-machine integration systems, which may significantly affect human behavior, emotions, or overall well-being.
- Development of algorithms, applications, or platforms capable of mobilizing public opinion or societal attitudes.
- Design and creation of automated decision-making systems, particularly those with high autonomy, catering to environments presenting clear safety or health concerns.
6. HOW IS EACH PROCEDURE IMPLEMENTED IN PRACTICE?
Please see the chart below for an overview of the entire procedure of science-tech ethics review:
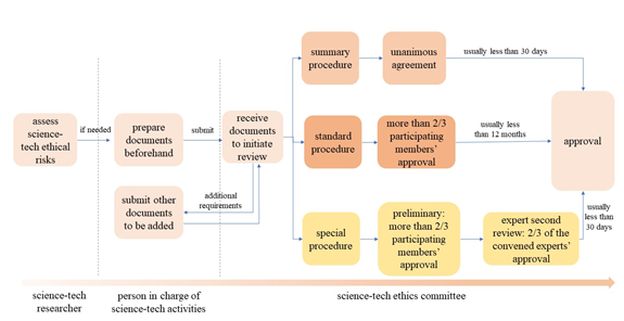
Additionally, the Measures establishes an emergency review procedure for situations like unexpected public emergencies. This streamlined process is designed to complete the science-tech ethical assessment within 72 hours. However, it's important to emphasize that the robustness of the ethical review standards remains unchanged, even under this expedited procedure.
7. WHAT CONSTITUTES THE CORE CRITERIA AND STANDARDS FOR SCIENCE-TECH ETHICS REVIEW?
Article 15 of the Measures highlights the key contents and standards of science-tech ethics review as summarized below:
- Principles: The proposed science-tech activities should balance innovation with risk management. They should prudently address uncertainties and technology application risks. Activities should align with ethical benchmarks, such as promoting human welfare, respecting life rights, ensuring fairness and transparency, and reasonably managing risks.
- Personnel: The qualifications of individuals involved in the science-tech activities should meet the required criteria.
- Infrastructure: The underlying research infrastructure and facility conditions should satisfy set standards.
- Value Proposition: The proposed activities should offer both scientific and societal value. The end goal should aim at fostering human well-being and sustainable societal advancements.
- Risk Management: Activities must have a rational risk-benefit profile. Ethical risk mitigation strategies and contingency plans should be in place and should be feasible.
- Conflict of Interest: The strategies set for declaring and managing conflicts of interest should be sound and justifiable.
Furthermore, the Measures specifies review priorities for different types of scientific endeavors:
- Human-centric Research: Fair participant recruitment, legal and ethical management of biological samples, proper processing of personal privacy data(including biometric information), reasonable remedial and protection plans, clear informed consent processes, and proper care for vulnerable populations are essential. Consent documents should be thorough, clearly articulated, and in full compliance with regulations.
- Animal-based Research: Activities should adhere to principles of replacement, reduction, and refinement (the 3Rs). The source and care of animals, including their housing and eventual disposition, should meet animal welfare standards. Adequate safety measures for both practitioners and the public should be in place.
- Data & Algorithm Research: Data processing activities and the development of new data technologies should align with national data security and personal information protection standards. Algorithms, models, and systems should respect principles like fairness, transparency, and reliability. Proper ethical risk assessments and user protection measures should be comprehensive and appropriate.
During the expert second review phase, the expert panel primarily focuses on the compliance and rationality of the findings from the preliminary review. This second review verifies whether the preliminary review adheres to China's laws, administrative regulations, relevant state rules, and the ethics of scientific and technological endeavors. It also ensures a thorough, appropriate, and rational assessment of potential ethical risks associated with science-tech activities and their corresponding preventive and control measures.
8. HOW IS EXTERNAL OVERSIGHT IMPLEMENTED FOR SCIENCE-TECH ETHICS REVIEW?
The National Science-Tech Ethics Management Information Registration Platform serves as the primary hub for regulatory bodies to supervise science-tech ethics reviews.
Upon the establishment of a science-tech ethics committee, organizations are legally required to register detailed information about the committee on the National Science-Tech Ethics Management Information Registration Platform within 30 days. The information required to be registered to the platform broadly includes the committee's structure, governing principles, working procedures, annual reports, and specifics about its members such as their credentials, roles, and experience in science-tech ethics.
Moreover, the platform is the designated location for the submission of the committee's annual reports. For science-tech activities that fall under Special Procedure—those included in the List—an additional registration post-approval is required, which includes the science-tech activity's action plan and findings from its ethical review and expert second review processes. Additionally, any changes in the registered information should be promptly updated, and the progress of the science-tech activities should be consistently reported.
While the platform is still under development, once fully operational, it's anticipated to seamlessly integrate with systems like the Public Service Platform of the National Science and Technology Information System, facilitating a more streamlined and collaborative approach to science-tech ethical governance and oversight.
Furthermore, an initiative is underway to introduce a certification mechanism for the science-tech ethics committee, fostering a culture of proactive adherence to science-tech ethics review certification among organizations.
9. ARE ENTITIES SUBJECT TO ADDITIONAL RESPONSIBILITIES UNDER THE SCIENCE-TECH MEASURES?
The Measures requires more than just ethics reviews. It also emphasizes the foundational role of organizational and systematic governance of science-tech activities within enterprises. Key provisions include:
- Designing and Refining Organizational Protocols: This includes structuring the science-tech ethics committee management policies, establishing guidelines for expedited ethics reviews during emergencies, and the procedures for oversight, confidentiality, and record-keeping. The goal is to ensure a structured and systematic ethics review process.
- Regular and Continuous Training: Such training includes tailored sessions for the science-tech ethics committee, especially training regarding emergency review scenarios, and broader educational initiatives for science-tech professionals, aiming to enhance their understanding and acumen in addressing ethical challenges in the rapidly evolving field.
CONCLUSION
The Measures lays out a lucid and coherent ethical review framework, streamlining the review procedures while upholding consistent standards. The refined ethical review framework will now emphasize ethical supervision in science-tech initiatives and highlights the importance of risk management. By prioritizing ethical compliance, the Measures seeks to safeguard the welfare of researchers, participants, and the public, and foster the sustainable development of scientific projects and advocate for responsible innovation. Looking forward, the evolving ethical review under the Measures are set to play a pivotal role in advancing China's scientific research and its ethical governance.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.


