The new coronavirus epidemic is becoming increasingly serious. As of February 21, 2020, the number of people infected with new coronavirus nationwide exceeded 70,000, and the number of deaths exceeded 2000. Medical workers in China and across the world are clinically testing multiple drugs to find or develop those which can effectively combat this virus.
A recent news has attracted everyone's attention – NEJM released a case about the first cure of a new type of coronavirus infection in the United States. It mentioned the use of Gilead's research on other coronaviruses such as Ebola virus. The drug Remdesivir seems to have a positive effect in treating patients.
According to the treatment process published in this case:
"When the U.S. infected person started hospitalization, his condition was relatively mild, so he used antipyretics such as paracetamol to alleviate the condition but did not achieve satisfactory results. The condition gradually worsened, and he started using vancomycin on the evening of the 6th day of hospitalization. He was treated with antibiotics such as cephalosporin, but his condition still did not show significant relief. Remdesivir was started on the evening of the seventh day, and antibiotics were discontinued. On the eighth day, his condition improved significantly, and he recovered after a few days." (First Case of 2019 Novel Coronavirus in the United States)
The drug is still in clinical trials and is being developed to treat other coronaviruses such as Ebola. The actual effects and side effects of the drug need further testing and observation. However, since there are currently no alternative treatments available, the possible effects of this drug has caused widespread interest.
Remdesivir is a nucleoside analogue developed by Gilead, a biotechnology company in the United States. It is mainly used to fight coronavirus. Its structural formula is as follows:
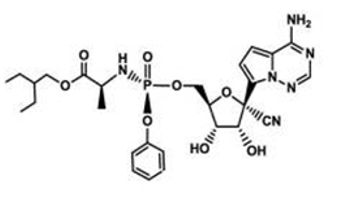
A glance at the patents under Gilead revealed a number of patents that may target Remdesivir. These patents protect drugs with the same structure as Remdesivir, and these patents point out that these drugs can be used to treat coronavirus. It can be judged that Gilead has made a patent portfolio for Remdesivir.
Patent contents reveal that Remdesivir is derived from a drug developed by Gilead around 2010 for the treatment of Paramyxoviridae. With continuous research and development, Gilead found that the substance can be used to treat coronavirus, and gradually identified the best chemical structure for treating coronavirus.
Gilead's patent portfolio around Remdesivir is shown in the following figure:
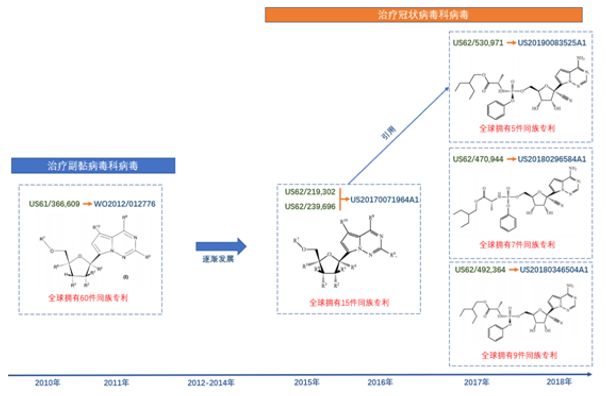
Note: The above figure only selects some key patents, not all patents are listed;
For the same family of patents, the earliest formal application is selected as an example, and different versions of the same application are included in the statistics.
The structural formulas disclosed in the three patents filed in 2017-2018 are no different from those of Remdesivir, and they can all be used to treat coronavirus.
The patent US20170071964A1 filed in 2015-2016 claims a compound that can treat coronaviruses and sandviruses, including a structure similar to Remdesivir.
Comparing US20170071964A1 with the patent file WO2012012776 filed by Gilead in 2011, the two patented compounds have many similarities in structure, and the inheritance and development relationship is obvious.
These patents are filed not only in the United States, but also in East Asia (including China, Korea, and Taiwan).
The clinical trial of the drug began on February 3 at the China-Japan Friendship Hospital. Regardless of whether the patent will become a factor affecting the application of the drug, this case reminds companies again that technology products are important, and the patent portfolio cannot be ignored. Protecting technical results is the proper intention of ensuring the advantages of tech products.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.


