The opportunity to decide writing this article is a startling photo that pop into my eyes when searching the meaning of the word "thalidomide" on the Internet. Thalidomide, the culprit of the famous "Contergan Event", was heard before. However, I didn't have a deeper understanding of the origin and development of the event until this time. After reading the information on the Internet and realizing the importance of the chiral compounds, I'd like to share some information about the novelty judgment of the patents for chiral compounds.
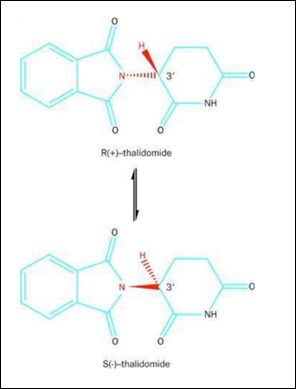
Thalidomide, having a chemical name of α-n-phthalidoglutamide, is a synthetic glutamate derivative.
(picture source: Baidu)
It is clear from the chemical formula of thalidomide that it is a typical chiral compound. So, what are the chiral compounds? According to the definition, chiral compounds refer to a kind of compounds whose stereoscopic structure and mirror image cannot coincide with each other. They are also called enantiomers.
Schematic diagram of chiral compounds (picture source: Baidu Encyclopedia)
As can be analogized with the structure and function of our hands, enantiomers of many chiral compounds have many same physical and chemical properties, such as melting point, solubility, participating in same type of chemical reaction. However, there are also enantiomers of many chiral compounds with great differences in physical and chemical properties, such as optical activity, odor, and they can interact with chiral substances to produce different products.
Before realizing the importance of the different properties of chiral compounds, there happened several medical events, and the most famous of them was the "Contergan Event".
In the year 1953, thalidomide was first synthesized by Ciba pharmaceutical factory, the predecessor of Novartis in Switzerland, when tried to develop antibiotics. In later tests, thalidomide was found to have no antibiotic activity, but it had a good sedative effect. Later, thalidomide was also synthesized by Chemie Grünenthal in West Germany and found to have a sedative effect on the central nervous system. Therefore, in the year 1957, Chemie Grünenthal launched the sedative to the European market under the banner of "no toxic and side effects". Its trade name is "Contergan". The manufacturer said that the drug could treat morning sickness, nausea and other pregnancy reactions without any side effects. It was good news for pregnant women at that time, which caused the frenzied purchase of pregnant women in Germany.

Thalidomide capsules sold in the market (picture source: Baidu)
Since 1960, however, there has been an abnormal increase in the birth defects rate in Europe. These babies have no arms and legs, with the hands and feet on the body, like seals' limbs, so they are called "seal deformed babies".
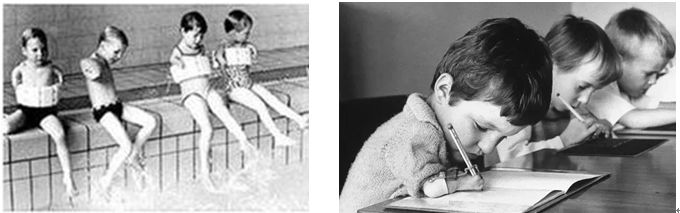
"seal deformed babies"(picture source: Baidu)
In the year 1961, an Australian obstetrician proposed that thalidomide was the main cause of infant deformity. So far, Chemie Grünenthal began to recall thalidomide, but the harm caused by thalidomide cannot be ignored. A paper published in 1988 stated that there were 3,049 infants with defects caused by thalidomide in Germany alone, and there were more than 10,000 infants affected by thalidomide worldwide.
In fact, the teratogenicity of thalidomide is due to the differences in the properties of chiral compounds. Thalidomide is a typical chiral compound, at the same time, it is also a racemic mixture, that is, an equal mixture of Levo-isomer (L-isomer) and corresponding Dextro-isomer (D-isomer). The L-isomer and the D-isomer of thalidomide have distinct functions: the D-isomer has central nervous sedative effect, while the L-isomer has a strong teratogenicity because it hinders the blood supply of pregnant women to the fetus. At that time, the drug took by the pregnant women were a mixture of thalidomide enantiomers. Generally, the purity of drugs should be at least 98%. Since thalidomide is a racemic mixture, it means that there were nearly 50% impurities in the Contergan at that time, which was certainly a fatal problem.
With the increasing recognition of the importance of the differences in the properties of chiral compounds, in the year 1992, the Center for Drug Evaluation and Development (CDER) of the U.S. Food and Drug Administration (FDA) published a development outline of optically active drugs. This policy requires drug developers to clearly quantify the pharmacodynamic and toxicological effects of each enantiomer in the instructions of new drugs, and when the two enantiomers have obvious different pharmacodynamic and toxicological effects, they must be marketed in the optically pure form. The decision of FDA has greatly promoted the research and development of chiral compounds, which has greatly changed the types of drug sold in the world market.
With the rapid development of chiral compounds, how can chiral compounds get better patent protection in China? Here, by introducing two Chinese patent applications that I met in my daily work, a brief summary of the general methods for judging the novelty of chiral compound patents in Chinese patent practice is made.
Novelty judgment: if the racemate of a compound has been disclosed in the prior art, and the compound has only one chiral center, the racemate is only an equimolar mixture of a pair of enantiomers. Generally speaking, a person skilled in the art will be able to split the R-isomer and S-isomer according to conventional technical means. At this time, it is presumed that the pair of enantiomers comprised in the compound has been disclosed, unless the applicant can prove that a person skilled in the art is unable to separate the enantiomers according to common skills.
Example 1: Chinese patent application CN102491956A, relating to "L-setastine hydrochloride and preparation method thereof", claim 1 thereof is listed as follows:
1. A compound of L-setastine hydrochloride of formula I of,
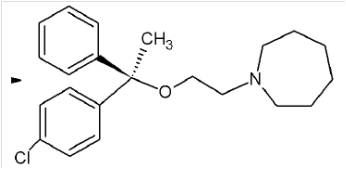
formula I.
During the substantive examination, the examiner used Document 1 (CN101486687A) as the closest prior art, and stated that as Document 1 has disclosed the racemate of setastine hydrochloride, claim 1 lacked novelty. However, there was no report on the resolution of setastine hydrochloride into isomers in the published literatures. The applicant attached the tests and results of the resolution of setastine hydrochloride by the conventional method, indicating that the chemical resolution method (chiral reagent), column chromatography, enzymolysis and crystal seed crystallization method cannot separate setastine. The main reason is that the position of N atom which can be used for resolution in its molecular structure is far away from the carbon atom in the chiral center: there are three carbon atoms and one oxygen atom, four atoms in total, therebetween. It is usually easier to make resolution when there are 1-2 atoms between the N atom used for resolution and the carbon atom in the chiral center. The examiner finally accepted the applicant's statement on the novelty of claim 1, and the patent application (CN102491956a) was finally granted.
Example 2: Chinese patent application (CN104892525A), relating to "Arylcyclopropylamine Based Demethylase Inhibitors of LSD1 and Their Medical Use". The claim 2 thereof is as follows:
"2. A compound, which is (-)(trans)-2-(4-(benzoxy)phenyl) cyclopropylamine."
During the substantive examination, the examiner pointed out
that claim 2 relating to the compound of
(-)(trans)-2-(4-(benzoxy)phenyl) cyclopropylamine. According
to paragraph [0017] of the specification, the absolute
configuration of (-) isomer is (1R, 2S). Document 1
(WO2010043721A1, 2010.04.22) discloses a compound
(trans)-2-[4-(Benzoxy)phenyl] cyclopropylamine (see paragraph 2 on
page 86 of the description of Document 1): 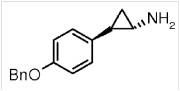 .At the same time, Document 1 also discloses
that all substances synthesized by its embodiment are mixtures of
two configurations (1R, 2S) and (1S, 2R) (see lines 21-22 on page
84 of the description of reference document 1). That is,
Document 1 discloses the racemate of (trans)-2-[4-(benzoxy)phenyl]
cyclopropylamine. Generally speaking, a person skilled in the
art will be able to split two isomers according to conventional
technical means. Therefore, it is presumed that a pair of
enantiomers contained in the substance disclosed in Document 1 has
been disclosed.
.At the same time, Document 1 also discloses
that all substances synthesized by its embodiment are mixtures of
two configurations (1R, 2S) and (1S, 2R) (see lines 21-22 on page
84 of the description of reference document 1). That is,
Document 1 discloses the racemate of (trans)-2-[4-(benzoxy)phenyl]
cyclopropylamine. Generally speaking, a person skilled in the
art will be able to split two isomers according to conventional
technical means. Therefore, it is presumed that a pair of
enantiomers contained in the substance disclosed in Document 1 has
been disclosed.
The applicant has made the following statement in observation: the most basic and well-known principle in the patent law is that a more general disclosure cannot destroy the novelty of claims involving specific embodiments. This principle is directly applicable to the case of the present application: claim 2 relates to a specific enantiomer, while Document 1 only provides a more general disclosure of the corresponding racemate. Therefore, Document 1 cannot destroy the novelty of claim 2. Claim 2, relating to the specific enantiomer (-) (trans)-2-[4-(benzoxy)phenyl] cyclopropylamine, possesses the novelty specified Article 22.2 of the Chinese patent law compared with the corresponding racemate of Document 1. However, in the subsequent examination, the examiner did not accept the applicant's statement about claim 2.
It can be seen that in the practice of patent examination in China, either the compound extracted and separated by the applicant has more than two chiral centers, or the compound has only one chiral center, but it can be proved that the compound cannot be separated by conventional technical means in the art, and no single left-handed compound and right-handed compound are found, otherwise it will be difficult to prove the novelty of the L-isomer and the D-isomer.
Concluding remarks:
The Royal Swedish Academy of Sciences announced on October 10, 2001 that half of the 2001 Nobel Prize in chemistry was awarded to American scientist William Knowles and Japanese scientist Ryoji Noyori for their contribution in the field of "chiral catalytic hydrogenation", and the other half was awarded to American scientist Barry Sharples for his contribution in the field of "chiral catalytic oxidation".

William Knowles Ryoji Noyori Barry Sharples
(picture source: Baidu)
It can be seen that more and more attention has been paid to the synthesis of chiral compounds in the fields of chemistry and medicine. With the rapid development of the synthesis of chiral compounds, the patent protection of chiral compounds is bound to attract more attention in the field of patent protection, which is also an opportunity and challenge for our practitioners to grasp and meet.
Reference:
Zheng XY., Chen ZY., Analysis of The Differences Between China and Europe In the Criteria for Judging the Novelty of Chiral Compounds [J]. Patent Agency. 2018, 02:52-58;
Wang J., Wen GY., Long QY., Patent Examination on Novelty and Inventiveness of Patent Application for Chiral Compounds [J]. Chinese invention and patent. 2016, 6, 84-87
Internet Article: Will the Ringleader after the Drug Teratogenicity of Tens of Thousands of Babies be the Answer to The Universe, Life and Everything?
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.

