The Central Drugs Standard Control Organization (CDSCO), New Delhi, under the aegis of the Ministry of Health & Family Welfare, initiated a nation-wide Pharmacovigilance Program of India (PvPI) in 2010. The PvPI, with the aim to safe guard public health in the country, has set-up Adverse drug reactions (ADRs) Monitoring Centres (AMCs) across the country. The AMCs, in order to monitor ADRs, occuring due to use of medicines, vaccines, medical devices, herbal products etc., collect ADRs reports from pharma companies, healthcare professionals and consumers/patients. They further evaluate/validate the same before forwarding the reports to Indian Pharmacopoeia Commission ("IPC") which is a National Coordinating Centre (NCC) for PvPI. The NCC analyses the PvPI database and raises alerts for risk-prone drugs to CDSCO. Further, NCC also shares this information with global ADR monitoring center (WHO-Uppsala Monitoring Centre (UMC)), Sweden, to contribute to the global ADRs data base.
NCC-PvPI identifies India centric drug safety signals after analyzing suspected ADRs and communicates its findings to CDSCO for appropriate regulatory action. Thereafter, the Subject expert committee (SEC) of CDSCO reviews the same and if in agreement, recommends the CDSCO to request all State/UT Drugs Controllers to instruct all the manufacturers licensed for the said product/drugs to include the ADRs in prescribing information (Package inserts and drug safety labels) of particular drugs. In case SEC is not satisfied with NCC-PvPI recommendations, they ask for more Individual Case Safety Reports/signals to support their recommendation. During 2019, in response to PvPI, the CDSCO issued a number of regulatory actions to all State/UTs drug controllers to direct the manufacturers of said tabulated formulations to incorporate details of ADR in the Prescribing information leaflet (PIL) of the drug marketed in the country. The year-end review summarizes the list of drug formulations with flagged ADRs1 :
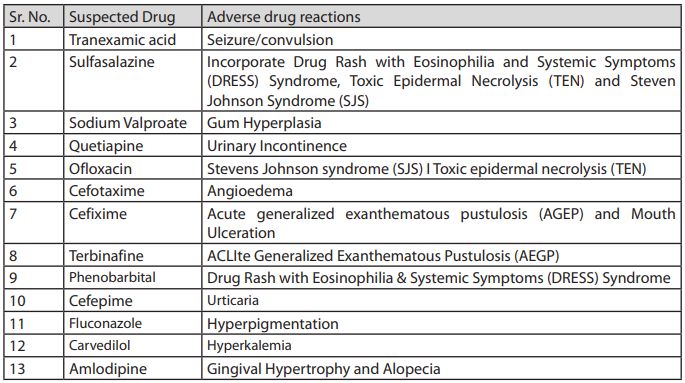
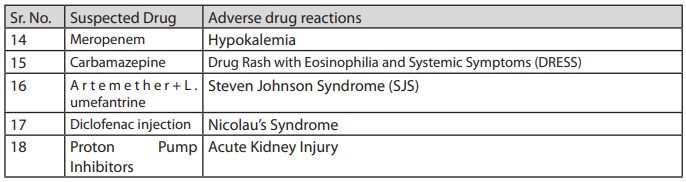
Note – Recently, NCC-PvPI was recognized as a WHO collaborating center for Pharmacovigilance in public health programs and health services due to its progressive contribution towards patient safety and wellbeing. At present 250 AMCs are active across the country. Of these centers, 21 AMCs play sentinel site for Revised National Tuberculosis Control Program (RNTCP), 20 for HIV control program on anti-retroviral Therapy (ART) and 6 are designated as Bedaquiline Cohort monitoring Centers.
Footnote
1. https://ipc.gov.in/mandates/pvpi/pvpi-updates/8-category-en/422-regulatory-actions.html
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.

